Ginsenoside Ra3CAS# 90985-77-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 90985-77-6 | SDF | Download SDF |
| PubChem ID | 73157064 | Appearance | Powder |
| Formula | C59H100O27 | M.Wt | 1241.4 |
| Type of Compound | Saponins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
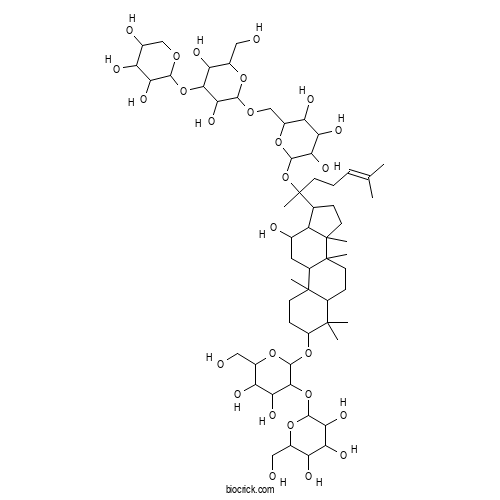
| Chemical Name | 2-[2-[[17-[2-[6-[[3,5-dihydroxy-6-(hydroxymethyl)-4-(3,4,5-trihydroxyoxan-2-yl)oxyoxan-2-yl]oxymethyl]-3,4,5-trihydroxyoxan-2-yl]oxy-6-methylhept-5-en-2-yl]-12-hydroxy-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)O)C)C)O)C)OC7C(C(C(C(O7)COC8C(C(C(C(O8)CO)O)OC9C(C(C(CO9)O)O)O)O)O)O)O)C | ||
| Standard InChIKey | QUNSGRLNZDSQJC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C59H100O27/c1-24(2)10-9-14-59(8,86-53-46(75)42(71)39(68)31(82-53)23-78-51-47(76)48(40(69)30(21-62)79-51)84-50-44(73)36(65)27(64)22-77-50)25-11-16-58(7)35(25)26(63)18-33-56(5)15-13-34(55(3,4)32(56)12-17-57(33,58)6)83-54-49(43(72)38(67)29(20-61)81-54)85-52-45(74)41(70)37(66)28(19-60)80-52/h10,25-54,60-76H,9,11-23H2,1-8H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ginsenoside Ra3 Dilution Calculator

Ginsenoside Ra3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8055 mL | 4.0277 mL | 8.0554 mL | 16.1108 mL | 20.1386 mL |
| 5 mM | 0.1611 mL | 0.8055 mL | 1.6111 mL | 3.2222 mL | 4.0277 mL |
| 10 mM | 0.0806 mL | 0.4028 mL | 0.8055 mL | 1.6111 mL | 2.0139 mL |
| 50 mM | 0.0161 mL | 0.0806 mL | 0.1611 mL | 0.3222 mL | 0.4028 mL |
| 100 mM | 0.0081 mL | 0.0403 mL | 0.0806 mL | 0.1611 mL | 0.2014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ginsenoside Rh8
Catalog No.:BCN0859
CAS No.:343780-69-8
- Ginsenoside F11
Catalog No.:BCN0858
CAS No.:115038-42-1
- Cirenshenoside S
Catalog No.:BCN0857
CAS No.:226572-11-8
- Ginsenoyne E
Catalog No.:BCN0856
CAS No.:126146-63-2
- Notoginsenoside T1
Catalog No.:BCN0855
CAS No.:343962-53-8
- Ciwujianoside C3
Catalog No.:BCN0854
CAS No.:114906-74-0
- Ciwujianoside A1
Catalog No.:BCN0853
CAS No.:120768-65-2
- Siraitic acid A
Catalog No.:BCN0852
CAS No.:183374-15-4
- Siraitic acid B
Catalog No.:BCN0851
CAS No.:183374-16-5
- 11-Deoxymogroside IIIE
Catalog No.:BCN0850
CAS No.:1793003-47-0
- 11-Oxomogroside I
Catalog No.:BCN0849
CAS No.:918972-06-2
- Mogroside II-B
Catalog No.:BCN0848
CAS No.:942615-25-0
- 1,3,5-tricaffeoylquinic acid
Catalog No.:BCN0861
CAS No.:1073897-80-9
- Heratomol
Catalog No.:BCN0862
CAS No.:61265-07-4
- Secologanin
Catalog No.:BCN0863
CAS No.:19351-63-4
- Clinoposaponin D
Catalog No.:BCN0864
CAS No.:1822328-43-7
- Clinoposaponin VIII
Catalog No.:BCN0865
CAS No.:152020-04-7
- Clinoposaponin X
Catalog No.:BCN0866
CAS No.:159122-00-6
- Clinoposaponin XI
Catalog No.:BCN0867
CAS No.:159122-01-7
- Clinoposaponin I
Catalog No.:BCN0868
CAS No.:152580-76-2
- Clinoposaponin IX
Catalog No.:BCN0869
CAS No.:159121-99-0
- Clinoposaponin VI
Catalog No.:BCN0870
CAS No.:152020-03-6
- Clinopodiside B
Catalog No.:BCN0871
CAS No.:155762-41-7
- Ginkgolic Acid C17:2
Catalog No.:BCN0872
CAS No.:102811-39-2
Characterization of the Components and Pharmacological Effects of Mountain-Cultivated Ginseng and Garden Ginseng Based on the Integrative Pharmacology Strategy.[Pubmed:33981239]
Front Pharmacol. 2021 Apr 26;12:659954.
Panax ginseng C. A. Mey (PGCAM) is a herbaceous perennial belonging to the Araliaceae family, mainly including Mountain-Cultivated Ginseng (MCG) and Garden Ginseng (GG) on the market. We aimed to establish a rapid, accurate and effective method to distinguish 15-year-old MCG and GG using ultra-performance liquid chromatography-quadrupole time-of-flight-tandem mass spectrometry (UPLC-QTOF-MS/MS), and also explored the pharmacological mechanisms of the main components using the Integrative Pharmacology-based Network Computational Research Platform of Traditional Chinese Medicine (TCMIP V2.0; http://www.tcmip.cn/). Altogether, 23 potential quality markers were characterized to distinguish 15-year-old MCG and GG, including ginsenosides Ra2, Rg1, and Ra1, and malonyl-Ginsenoside Ra3, etc. The contents of 19 constituents (mainly protopanaxadiol-type) were higher in MCG compared with that in GG, and four constituents (mainly carbohydrate compounds) were higher in GG. The 105 putative targets corresponding to 23 potential quality markers were mainly involved in 30 pathways, which could be divided into 10 models, such as immune regulation, systems (metabolic, nervous, cardiovascular, reproductive), blood-pressure regulation, as well as antitumor, antiaging, antibacterial and anti-inflammatory effects. Furthermore, the potential quality markers of MCG and GG could inhibit the proliferation of breast cancer by regulating the mRNA expression of PSA, S6K, MDM2, and P53 genes by acting on AR, MTOR, PI3K and other targets. The Integrative Pharmacology Strategy may provide an efficient way to identify chemical constituents and explore the pharmacological actions of TCM formulations.
Identification of mountain-cultivated ginseng and cultivated ginseng using UPLC/oa-TOF MSE with a multivariate statistical sample-profiling strategy.[Pubmed:27746686]
J Ginseng Res. 2016 Oct;40(4):344-350.
BACKGROUND: Mountain-cultivated ginseng (MCG) and cultivated ginseng (CG) both belong to Panax ginseng and have similar ingredients. However, their pharmacological activities are different due to their significantly different growth environments. METHODS: An ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-QTOF-MS/MS)-based approach was developed to distinguish MCG and CG. Multivariate statistical methods, such as principal component analysis and supervised orthogonal partial-least-squares discrimination analysis were used to select the influential components. RESULTS: Under optimized UPLC-QTOF-MS/MS conditions, 40 ginsenosides in both MCG and CG were unambiguously identified and tentatively assigned. The results showed that the characteristic components of CG and MCG included Ginsenoside Ra3/isomer, gypenoside XVII, quinquenoside R1, ginsenoside Ra7, notoginsenoside Fe, ginsenoside Ra2, ginsenoside Rs6/Rs7, malonyl ginsenoside Rc, malonyl ginsenoside Rb1, malonyl ginsenoside Rb2, palmitoleic acid, and ethyl linoleate. The malony ginsenosides are abundant in CG, but higher levels of the minor ginsenosides were detected in MCG. CONCLUSION: This is the first time that the differences between CG and MCG have been observed systematically at the chemical level. Our results suggested that using the identified characteristic components as chemical markers to identify different ginseng products is effective and viable.
Chemical differentiation and quality evaluation of commercial Asian and American ginsengs based on a UHPLC-QTOF/MS/MS metabolomics approach.[Pubmed:25448530]
Phytochem Anal. 2015 Mar-Apr;26(2):145-60.
INTRODUCTION: Asian and American ginsengs are widely used medicinal materials and are being used more and more in health products. The two materials look alike but function differently. Various forms of both types of ginseng are found in the market, causing confusion for consumers in their choice. OBJECTIVE: To evaluate the overall quality of commercial Asian and American ginsengs and investigate the characteristic chemical markers for differentiating between them. METHODS: This article investigated 17 Asian and 21 American ginseng samples using an ultra-HPLC combined with quadrupole time-of-flight MS/MS technique. The data were processed by principal component analysis and orthogonal partial least squared discriminant analysis. RESULTS: In the chromatograms, a total of 40 peaks were detected. Among them, six were positively identified, and all of the remainder were tentatively identified. According to statistical results, ginsenosides Rf, Rb2 and Rc together with their isomers and derivatives were more likely to be present in Asian ginsengs, whereas ginsenoside Rb1 , pseudoginsenoside F11 and ginsenoside Rd together with their isomers and derivatives tended to be present in American ginsengs. For Asian ginsengs, Ginsenoside Ra3 and 20-beta-D-glucopyranosyl-ginsenoside-Rf were more likely to be present in forest samples, whereas contents of floralquinquenoside B, ginsenosides Ro and Rc, and zingibroside R1 were higher in sun-dried ginsengs. For American ginseng, wild samples often had more of the notoginsenosides R1 and Rw2 and less of the ginsenosides Rd, Rd isomer and 20 (S)-Rg3 than cultivated samples. CONCLUSION: The method provided important fingerprint information for authentication and evaluation of Asian and American ginsengs from various commercial products.
[Chemical constituents from processed rhizomes of Panax notoginseng].[Pubmed:24558875]
Zhongguo Zhong Yao Za Zhi. 2013 Nov;38(22):3910-7.
To investigate the chemical constituents of the processed rhizomes of Panax notoginseng, their 70% ethanol extract was chromatographed on macroporous resin (SP825), silica gel, RP-C18 and semi-preparative HPLC to afford compounds 1-23. On the basis of physicochemical properties and spectral data analysis, their structures were identified to be 6'-O-Acetylginsenoside Rh1 (1), ginsenoside RK3 (2), ginsenoside Rh4 (3), 20S-ginsenoside Rg3 (4), ginsenoside Rk1 (5), 20R-ginsenoside Rg3 (6), ginsenoside Rg5 (7), ginsenoside F2 (8), 20S-ginsenoside Rh1 (9), 20R-ginsenoside Rh1 (10), gypenoside X VII (11), notoginsenoside Fa, (12), Ginsenoside Ra3 (13), ginsenoside Rg1 (14), ginsenoside Re (15), notoginsenoside R2 (16), ginsenoside Rg2 (17), notoginsenoside R1 (18), ginsenoside Rd (19), ginsenoside Rb1 (20), notoginsenoside D (21), notoginsenoside R4 (22) and ginsenoside Rb2 (23), respectively. Among them, compound 1 was isolated from P. notoginseng for the first time, and compounds 4, 6, 8 and 11 were isolated from the processed P. notoginseng for the first time. According to the fingerprint profiles of raw and processed P. notoginseng, the putative chemical conversion pathways of panoxatriol and panoxadiol compounds in the processing procedure was deduced, and the results revealed the main reactions to be dehydration and glycosyl hydrolysis.
[Chemical constituents from roots and rhizomes of Panax ginseng cultivated in Jilin province].[Pubmed:24380303]
Zhongguo Zhong Yao Za Zhi. 2013 Sep;38(17):2807-17.
The chemical constituents of the roots and rhizomes of Panax ginseng were systematically investigated by various column chromatographic methods including Amberlite XAD-4 macroporous adsorptive resins and silica gel as well as high-performance liquid chromatography, and their chemical structures were identified by physico-chemical properties and spectral analyses. Twenty-eight compounds were isolated from the 70% ethanolic-aqueous extract and identified as koryoginsenoside R1 (1), ginsenoside Rg1 (2), ginsenoside Rf (3), notoginsenoside R2 (4), ginsenoside Rg2 (5), notoginsenoside Fe (6), ginsenjilinol (7), ginsenoside Re5 (8), noto-ginsenoside N (9), notoginsenoside R1 (10), ginsenoside Re2 (11), ginsenoside Re1 (12), ginsenoside Re (13), ginsenoside Rs2 (14), ginsenoside Ro methyl ester (15), ginsenoside Rd (16), ginsenoside Re3 (17), ginsenoside Re4 (18), 20-gluco-ginsenoside Rf (19), ginsenoside Ro (20), ginsenoside Rc (21), quinquenoside-R1 (22), ginsenoside Ra2 (23), ginsenoside Rb1 (24), ginsenoside Ra1 (25), Ginsenoside Ra3 (26), ginsenoside Rb2 (27), and notoginsenoside R4 (28). All isolated compounds are 20 (S) -protopanaxadiol or protopanaxatriol type triterpenoid saponins. Compound 1 was isolated from the roots and rhizomes of P. ginseng cultivated in Jilin province for the first time and compound 6 was isolated from the roots and rhizomes of P. ginseng for the first time. The 1H-NMR data of compounds 6, 14 and 19 were assigned for the first time.
Isolation and characterization of a new ginsenoside from the fresh root of Panax Ginseng.[Pubmed:20428044]
Molecules. 2010 Mar 30;15(4):2319-25.
A new saponin, malonylGinsenoside Ra3, was isolated from the fresh root of Panax ginseng, along with four known ginsenosides. The new compound was identified as (20S)-protopanaxadiol-3-O-(6-O-malonyl-beta-D-glucopyranosyl(1-->2)-beta-D-glucop yranoside-20-O-beta-D-xylopyranosyl(1-->3)-beta-D-glucopyranosyl(1-->6)-beta-D-gl ucopyranoside on the basis of extensive 1D and 2D NMR as well as HRESI-MS spectroscopic data analysis.


