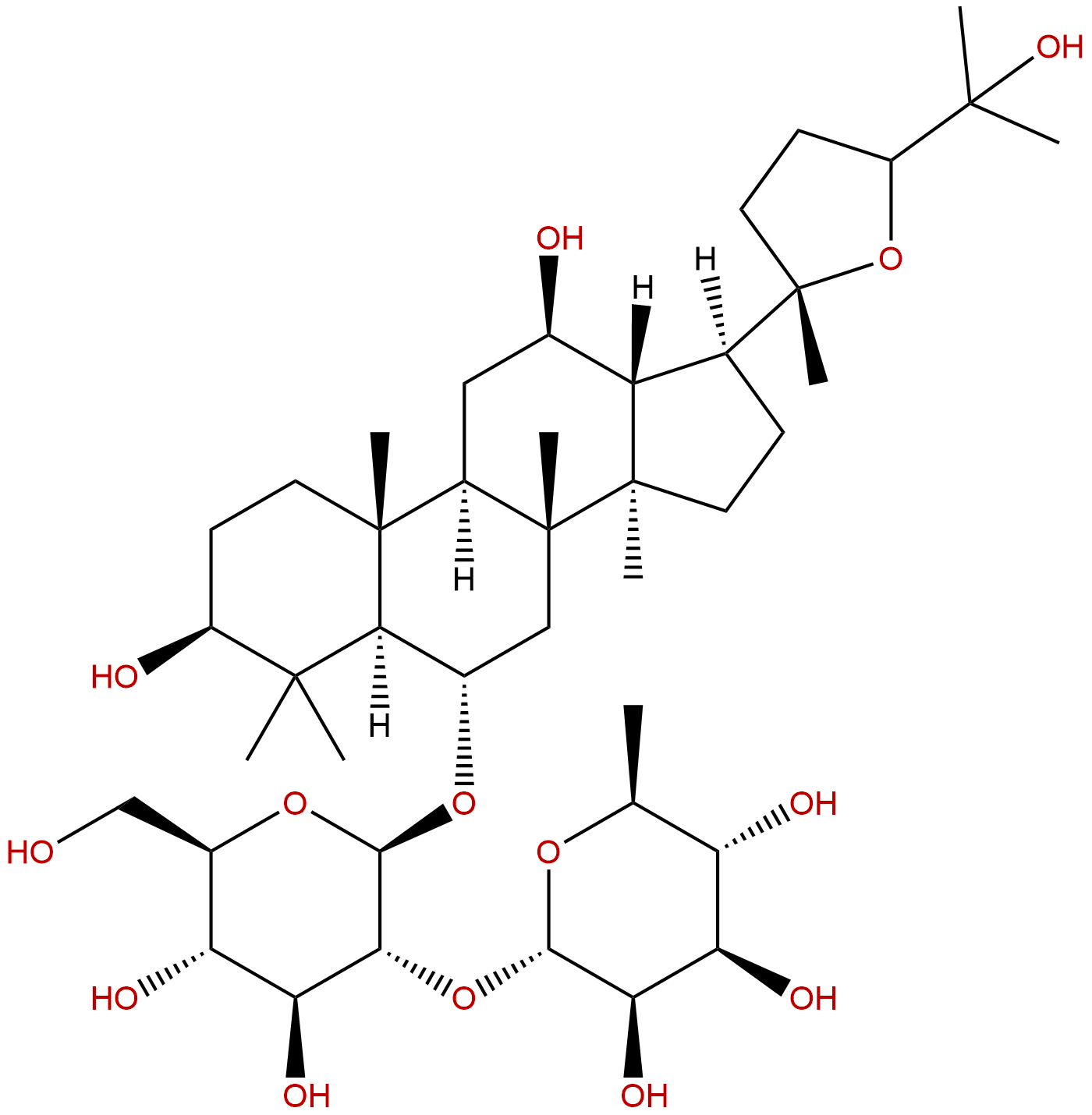
Ginsenoside F11CAS# 115038-42-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 115038-42-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C42H72O14 | M.Wt | 801 |
| Type of Compound | Saponins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ginsenoside F11 Dilution Calculator

Ginsenoside F11 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2484 mL | 6.2422 mL | 12.4844 mL | 24.9688 mL | 31.211 mL |
| 5 mM | 0.2497 mL | 1.2484 mL | 2.4969 mL | 4.9938 mL | 6.2422 mL |
| 10 mM | 0.1248 mL | 0.6242 mL | 1.2484 mL | 2.4969 mL | 3.1211 mL |
| 50 mM | 0.025 mL | 0.1248 mL | 0.2497 mL | 0.4994 mL | 0.6242 mL |
| 100 mM | 0.0125 mL | 0.0624 mL | 0.1248 mL | 0.2497 mL | 0.3121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cirenshenoside S
Catalog No.:BCN0857
CAS No.:226572-11-8
- Ginsenoyne E
Catalog No.:BCN0856
CAS No.:126146-63-2
- Notoginsenoside T1
Catalog No.:BCN0855
CAS No.:343962-53-8
- Ciwujianoside C3
Catalog No.:BCN0854
CAS No.:114906-74-0
- Ciwujianoside A1
Catalog No.:BCN0853
CAS No.:120768-65-2
- Siraitic acid A
Catalog No.:BCN0852
CAS No.:183374-15-4
- Siraitic acid B
Catalog No.:BCN0851
CAS No.:183374-16-5
- 11-Deoxymogroside IIIE
Catalog No.:BCN0850
CAS No.:1793003-47-0
- 11-Oxomogroside I
Catalog No.:BCN0849
CAS No.:918972-06-2
- Mogroside II-B
Catalog No.:BCN0848
CAS No.:942615-25-0
- Mogroside I-A1
Catalog No.:BCN0847
CAS No.:88901-46-6
- Mogroside I-E1
Catalog No.:BCN0846
CAS No.:88901-39-7
- Ginsenoside Rh8
Catalog No.:BCN0859
CAS No.:343780-69-8
- Ginsenoside Ra3
Catalog No.:BCN0860
CAS No.:90985-77-6
- 1,3,5-tricaffeoylquinic acid
Catalog No.:BCN0861
CAS No.:1073897-80-9
- Heratomol
Catalog No.:BCN0862
CAS No.:61265-07-4
- Secologanin
Catalog No.:BCN0863
CAS No.:19351-63-4
- Clinoposaponin D
Catalog No.:BCN0864
CAS No.:1822328-43-7
- Clinoposaponin VIII
Catalog No.:BCN0865
CAS No.:152020-04-7
- Clinoposaponin X
Catalog No.:BCN0866
CAS No.:159122-00-6
- Clinoposaponin XI
Catalog No.:BCN0867
CAS No.:159122-01-7
- Clinoposaponin I
Catalog No.:BCN0868
CAS No.:152580-76-2
- Clinoposaponin IX
Catalog No.:BCN0869
CAS No.:159121-99-0
- Clinoposaponin VI
Catalog No.:BCN0870
CAS No.:152020-03-6
Comprehensive investigation on metabolites of Panax quinquefolium L. in two main producing areas of China based on ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry.[Pubmed:34905806]
J Mass Spectrom. 2021 Nov 5;56(12):e4791.
Jilin Province and Shandong Province are two main American ginseng (AG) producing areas in China. The geographical difference existed in these two provinces. Aiming at evaluating the similarities and differences of the secondary metabolites, the comprehensive metabolite profiling of AG from Jilin Province (AGJ ) and Shandong Province (AGS ) was performed based on UPLC-QTOF-MS for the first time. In screening analysis, a total of 111 shared compounds, with ginsenosides being major components, were identified or tentatively characterized, which indicated that AGJ and AGS were all rich in phytochemicals and contained similar structural types. Untargeted metabolomics analysis indicated that there were significant differences in the contents of certain constituents in AGJ and AGS . Nineteen (12 for AGJ , 7 for AGS ) potential producing area-dependent chemical markers were discovered. Based on the contents and MS responses, ginsenoside Rg1 , Re, and pseudoGinsenoside F11 could be considered as the characteristical markers of AGJ , whereas ginsenoside Rg3 and Rh2 of AGS . This comprehensive phytochemical profile study could provide valuable chemical evidence for evaluating the characteristics qualities of AG from various producing areas.
Simultaneous quantitative assays of 15 ginsenosides from 119 batches of ginseng samples representing 12 traditional Chinese medicines by ultra-high performance liquid chromatography coupled with charged aerosol detector.[Pubmed:34487881]
J Chromatogr A. 2021 Oct 11;1655:462504.
Despite the extensive consumption of ginseng, precise quality control of different ginseng products is highly challenging due to the containing of ginsenosides in common for different Panax species or different parts (e.g. root, leaf, and flower) of a same species. Herein we performed a comparative investigation of diverse ginseng products by simultaneously assaying 15 saponins (notoginsenoside R1, ginsenosides Rg1, -Re, -Rf, -Ra2, -Rb1, -Rc, -Ro, -Rb2, -Rb3, -Rd, 20(R)-ginsenoside Rg3, 24(R)-pseudoGinsenoside F11, chikusetsusaponins IV, and -IVa) using an ultra-high-performance liquid chromatography/charged aerosol detector (UHPLC-CAD) approach. Twelve Panax-derived ginseng products (involving P. ginseng root, P. quinquefolius root, P. notoginseng root, Red ginseng, P. ginseng leaf, P. quinquefolius leaf, P. notoginseng leaf, P. ginseng flower, P. quinquefolius flower, P. notoginseng flower, P. japonicus root, and P. japonicus var. major root) were considered. Benefiting from the condition optimization, the baseline resolution of 15 ginsenosides was achieved on a CORTECS UPLC Shield RP18 column. This method was validated as specific, precise (0.81-1.94% intra-day variation; 0.86-2.35% inter-day variation), and accurate (recovery: 90.73-107.5%), with good linearity (R(2) > 0.999), high sensitivity (limit of detection: 0.02-0.21 mug; limit of quantitation: 0.04-0.42 mug) and sample stability (1.49-4.74%). Its application to 119 batches of ginseng samples unveiled vital information enabling the authentication of these different ginseng products. Detection of ginsenosides by CAD exhibited superiority over UV in sensitivity and the ability to monitor chromophore-free structures. Large-scale comparative studies by quantifying multiple markers provide methodological reference to the precise quality control of herbal medicine.
Terahertz Spectroscopy for Accurate Identification of Panax quinquefolium Basing on Nonconjugated 24(R)-Pseudoginsenoside F11.[Pubmed:33860277]
Plant Phenomics. 2021 Jan 27;2021:6793457.
Panax quinquefolium is a perennial herbaceous plant that contains many beneficial ginsenosides with diverse pharmacological effects. 24(R)-pseudoGinsenoside F11 is specific to P. quinquefolium, a useful biomarker for distinguishing this species from other related plants. However, because of its nonconjugated property and the complexity of existing detection methods, this biomarker cannot be used as the identification standard. We herein present a stable 24(R)-pseudoGinsenoside F11 fingerprint spectrum in the terahertz band, thereby proving that F11 can be detected and quantitatively analyzed via terahertz spectroscopy. We also analyzed the sample by high-performance liquid chromatography-triple quadrupole mass spectrometry. The difference between the normalized data for the two analytical methods was less than 5%. Furthermore, P. quinquefolium from different areas and other substances can be clearly distinguished based on these terahertz spectra with a standard principal component analysis. Our method is a fast, simple, and cost-effective approach for identifying and quantitatively analyzing P. quinquefolium.
Quantitative aspects of the hydrolysis of ginseng saponins: Application in HPLC-MS analysis of herbal products.[Pubmed:33841005]
J Ginseng Res. 2021 Mar;45(2):246-253.
BACKGROUND: Ginseng is one of the most valuable herbal supplements. It is challenging to perform quality control of ginseng products due to the diversity of bioactive saponins in their composition. Acid or alkaline hydrolysis is often used for the structural elucidation of these saponins and sugars in their side chains. Complete transformation of the original ginsenosides into their aglycones during the hydrolysis is one of the ways to determine a total saponin group content. The main hurdle of this approach is the formation of various by-products that was reported by many authors. METHODS: Separate HPLC assessment of the total protopanaxadiol, protopanaxatriol and ocotillol ginsenoside contents is a viable alternative to the determination of characteristic biomarkers of these saponin groups, such as ginsenoside Rf and pseudoGinsenoside F11, which are commonly used for authentication of P. ginseng Meyer and P. quinquefolius L. samples respectively. Moreover, total ginsenoside content is an ideal aggregated parameter for standardization and quality control of ginseng-based medicines, because it can be directly applied for saponin dosage calculation. RESULTS: Different hydrolysis conditions were tested to develop accurate quantification method for the elucidation of total ginsenoside contents in herbal products. Linearity, limits of quantification, limits of detection, accuracy and precision were evaluated for the developed HPLC-MS method. CONCLUSION: Alkaline hydrolysis results in fewer by-products than sugar elimination in acidic conditions. An equimolar response, as a key parameter for quantification, was established for several major ginsenosides. The developed approach has shown acceptable results in the analysis of several different herbal products.
Pseudoginsenoside F11 ameliorates the dysfunction of the autophagy-lysosomal pathway by activating calcineurin-mediated TFEB nuclear translocation in neuron during permanent cerebral ischemia.[Pubmed:33422553]
Exp Neurol. 2021 Apr;338:113598.
We have previously found that transcription factor EB (TFEB), as a master regulator of autophagy and lysosome biogenesis, provides neuroprotective effects on cerebral ischemia-induced neuronal damage by activation of autophagy-lysosomal pathway (ALP). We have also reported that PseudoGinsenoside F11 (PF11), an ocotillol-type saponin isolated from Panax quinquefolium L., significantly attenuates the ischemic injury of rats subjected to permanent middle cerebral artery occlusion (pMCAO), possibly by alleviating the autophagic/lysosomal defects. The present study aims to investigate whether the beneficial effect of PF11 on ALP dysfunction induced by permanent ischemic stroke is based on its regulation of TFEB nuclear translocation in pMCAO rats and the oxygen-glucose-deprived (OGD) primary neurons. Meanwhile, the role of calcineurin, a serine/threonine protein phosphatase, during this process in which PF11 regulated TFEB transcriptional activity was also explored. The data showed that PF11 exerted significant protective effects on pMCAO-induced injury and decreased OGD-induced neuronal death. The nuclear localization of TFEB was decreased at 24 h after pMCAO. Notably, PF11 (6, 12 mg/kg, i.v.) significantly increased TFEB nuclear expression and Tfeb mRNA level at 24 h following pMCAO. OGD treatment promoted TFEB aggregation and nuclear translocation until 6 h, and the nuclear localization of TFEB was decreased at 12 h. Similarly, PF11 (30, 100 muM) could also promote the translocation of TFEB into nuclear in primary neurons at 12 h after OGD treatment. Moreover, PF11 attenuated OGD-induced lysosomal dysfunction and abnormal accumulation of autophagosomes and substrates. These in vitro effects could be abolished by neuronal-specific knocking down of TFEB via transfecting primary neurons with lentivirus encoding shTfeb. Further studies indicated that cyclosporine (10 muM), an inhibitor of calcineurin, could significantly diminish the effects of PF11 on TFEB nuclear translocation and ALP dysfunction in OGD-treated neurons. In summary, these results demonstrate that PF11 attenuates the dysfunction of ALP in permanent cerebral ischemia by promoting the calcineurin-mediated nuclear translocation of TFEB and further identifies an autophagic mechanism of PF11 against cerebral ischemia.
Panax quinquefolium saponin liposomes prepared by passive drug loading for improving intestinal absorption.[Pubmed:32996345]
Drug Dev Ind Pharm. 2020 Oct;46(10):1684-1694.
Panax quinquefolium saponin (PQS) composed of 45% pseudo-Ginsenoside F11 (PF11), is a natural mixture of sterol compounds obtained from the American ginseng plant, having numerous promising benefits for health. However, low solubility and permeability limit the development of PQS as a therapeutic agent for oral administration. In this study, PQS liposomes (PQS-Lips) were prepared by thin layer hydration, an in situ single-pass intestinal perfusion (SPIP) model was used to verify the improvement of membrane permeability of PQS-Lips. PQS-Lips had a high encapsulation efficiency (EE) of 65% approximately 70%, a particle size about 100.0 nm, and a zeta potential of -60 mV with regular spherical surface. FTIR and DSC showed the PQS in liposomes were amorphous, indicating that hydrogen bonds formed between one or several hydroxyl groups in PQS and C-O group at the phospholipid polar terminal. In addition, PQS-Lips showed sustained release in vitro than PQS at pH 1.2 and pH 6.8, and PQS-Lips had good stability in simulated gastric and intestinal fluid. Then, the absorption rate (K a) and effective permeability coefficient (P eff) of PQS-Lips in the whole small intestine were significantly higher than those in PQS solution (PQS-Sol), which proved that the PQS-Lips could significantly increase the membrane permeability of PQS and promote its absorption in the small intestine. From the experimental results, it could be known that liposome technology could effectively improve the absorption of PQS in the small intestine.
The metabolites and biotransformation pathways in vivo after oral administration of ocotillol, RT5 , and PF11.[Pubmed:32307731]
Biomed Chromatogr. 2020 Aug;34(8):e4856.
Ocotillol, pseudo-ginsenoside RT5 (RT5 ), and pseudo-Ginsenoside F11 (PF11 ) are ocotillol-type saponins that have the same aglycone structure but with different numbers of glucose at the C-6 position. In this study, the metabolites of ocotillol, RT5 , and PF11 in rat plasma, stomach, intestine, urine, and feces after oral administration were investigated by ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry. The results showed that RT5 was easily biotransformed into metabolites in vivo, whereas PF11 and RT5 were difficult to be biotransformed. Hydrogenation, dehydrogenation, dehydration, deglycosylation, deoxygenation, hydration, phosphorylation, deoxidation, glucuronidation, and reactions combining amino acid were speculated to be involved in the biotransformation of ocotillol, RT5 , and PF11 . Based on the structural analysis of metabolites, it was deduced that hydrogenation, dehydration, deoxidation, and reactions combining amino acid occurred on the aglycone structure, whereas deglycosylation, hydration, and phosphorylation occurred on the glycosyl chain. Further, metabolites in plasma, urine, feces, and tissues were different: First, glucuronidation products were found in urine, stomach, intestine, and feces, but not in plasma. Second, the ocotillol prototype was not identified in urine samples. Third, the RT5 prototype was found in stomach, intestine, feces, and urine, but not in plasma.


