ConfusarinCAS# 108909-02-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108909-02-0 | SDF | Download SDF |
| PubChem ID | 11983285.0 | Appearance | Powder |
| Formula | C17H16O5 | M.Wt | 300.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
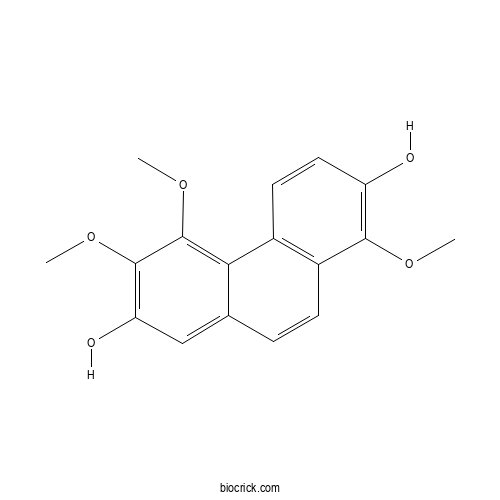
| Chemical Name | 1,5,6-trimethoxyphenanthrene-2,7-diol | ||
| SMILES | COC1=C(C=CC2=C1C=CC3=CC(=C(C(=C32)OC)OC)O)O | ||
| Standard InChIKey | JHNVCKNCEVZGGC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16O5/c1-20-15-11-5-4-9-8-13(19)16(21-2)17(22-3)14(9)10(11)6-7-12(15)18/h4-8,18-19H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Confusarin Dilution Calculator

Confusarin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3299 mL | 16.6495 mL | 33.2989 mL | 66.5978 mL | 83.2473 mL |
| 5 mM | 0.666 mL | 3.3299 mL | 6.6598 mL | 13.3196 mL | 16.6495 mL |
| 10 mM | 0.333 mL | 1.6649 mL | 3.3299 mL | 6.6598 mL | 8.3247 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.6649 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Denthyrsinin
Catalog No.:BCX1497
CAS No.:118169-17-8
- 9-epi-6-Methoxygeniposidic acid
Catalog No.:BCX1496
CAS No.:1215178-87-2
- 12β-Acetoxycoccinic acid
Catalog No.:BCX1495
CAS No.:125247-74-7
- 11-O-Syringylbergenin
Catalog No.:BCX1494
CAS No.:126485-47-0
- 7,4'-Di-O-methyltectorigenin
Catalog No.:BCX1493
CAS No.:13186-08-8
- Ginsenoside CY
Catalog No.:BCX1492
CAS No.:83480-65-3
- ent-kauran-16β,17-diol
Catalog No.:BCX1491
CAS No.:16836-31-0
- Erucic acid
Catalog No.:BCX1490
CAS No.:112-86-7
- Dihydrohelenalin
Catalog No.:BCX1489
CAS No.:34257-95-9
- 12-Hydroxyganoderenic acid B
Catalog No.:BCX1488
CAS No.:1309931-84-7
- Paroxetine Impurity A Hydrochloride salt
Catalog No.:BCX1487
CAS No.:1394842-91-1
- Fluvoxamine Impurity D
Catalog No.:BCX1486
CAS No.:61718-80-7
- Swertiaside
Catalog No.:BCX1499
CAS No.:96087-14-8
- Potentillanoside A
Catalog No.:BCX1500
CAS No.:1309589-79-4
- Homocapsaicin II
Catalog No.:BCX1501
CAS No.:71240-51-2
- Regaloside E
Catalog No.:BCX1502
CAS No.:123134-21-4
- Hydroxylinderstrenolide
Catalog No.:BCX1503
CAS No.:20267-92-9
- Visamminol-3'-O-glucoside
Catalog No.:BCX1504
CAS No.:2254096-98-3
- Tetrahydrodehydrodiconiferyl alcohol
Catalog No.:BCX1505
CAS No.:5234-70-8
- Fulvotomentoside A
Catalog No.:BCX1506
CAS No.:150107-44-1
- Sarsasapogenin acetate
Catalog No.:BCX1507
CAS No.:35319-91-6
- 4'-Hydroxyflavone
Catalog No.:BCX1508
CAS No.:4143-63-9
- Isoemetine hydrobromide
Catalog No.:BCX1509
CAS No.:21026-77-7
- Isocoptisine acetate
Catalog No.:BCX1510
CAS No.:30426-66-5
Discovery of inhibitors of protein tyrosine phosphatase 1B contained in a natural products library from Mexican medicinal plants and fungi using a combination of enzymatic and in silico methods*.[Pubmed:38027024]
Front Pharmacol. 2023 Oct 31;14:1281045.
This work aimed to discover protein tyrosine phosphatase 1B (PTP1B) inhibitors from a small molecule library of natural products (NPs) derived from selected Mexican medicinal plants and fungi to find new hits for developing antidiabetic drugs. The products showing similar IC(50) values to ursolic acid (UA) (positive control, IC(50) = 26.5) were considered hits. These compounds were canophyllol (1), 5-O-(beta-D-glucopyranosyl)-7-methoxy-3',4'-dihydroxy-4-phenylcoumarin (2), 3,4-dimethoxy-2,5-phenanthrenediol (3), masticadienonic acid (4), 4',5,6-trihydroxy-3',7-dimethoxyflavone (5), E/Z vermelhotin (6), tajixanthone hydrate (7), quercetin-3-O-(6''-benzoyl)-beta-D-galactoside (8), lichexanthone (9), melianodiol (10), and Confusarin (11). According to the double-reciprocal plots, 1 was a non-competitive inhibitor, 3 a mixed-type, and 6 competitive. The chemical space analysis of the hits (IC(50) < 100 muM) and compounds possessing activity (IC(50) in the range of 100-1,000 muM) with the BIOFACQUIM library indicated that the active molecules are chemically diverse, covering most of the known Mexican NPs' chemical space. Finally, a structure-activity similarity (SAS) map was built using the Tanimoto similarity index and PTP1B absolute inhibitory activity, which allows the identification of seven scaffold hops, namely, compounds 3, 5, 6, 7, 8, 9, and 11. Canophyllol (1), on the other hand, is a true analog of UA since it is an SAR continuous zone of the SAS map.
Recent Research Progress on Natural Stilbenes in Dendrobium Species.[Pubmed:36364058]
Molecules. 2022 Oct 25;27(21):7233.
Dendrobium is the second biggest genus in the Orchidaceae family, and many of them have been utilized as a traditional Chinese medicine (TCM) for thousands of years in China. In the last few decades, constituents with great chemical diversity were isolated from Dendrobium, and a wide range of biological activities were detected, either for crude extracts or for pure compounds. Stilbene compound is one of the primary active constituents in the genus Dendrobium. At present, 267 stilbene compounds with clarified molecular structures have been extracted and isolated from 52 species of Dendrobium, including 124 phenanthrenes and 143 bibenzyls. At the same time, activity studies have indicated that 157 compounds have pharmaceutical activity. Among them, most of the compounds showed antitumor activity, followed by antioxidant, anti-inflammatory and anti-alpha-glucosidase inhibitory activities. Additionally, 54 compounds have multiple pharmacological activities, such as Confusarin (14), 2,4,7-trihydroxy-9,10-dihydro-phenanthrene (43), moscatilin (148), gigantol (150) and batatasin III (151). This review summarizes current knowledge about the chemical composition of stilbene, bioactivities and pharmacologic effects in 52 species of Dendrobium. We also expect to provide a reference for further research, development and utilization of stilbene constituents in the Dendrobium genus.
A Simple Method for the Screening and Measurement of Phenols in Dendrobium chrysotoxum by a Miniature Mass Detector Using a Matrix Solid-Phase Dispersion Method.[Pubmed:30809414]
J Anal Methods Chem. 2019 Jan 23;2019:6737632.
The present study aims at building a miniature mass method for the simultaneous determination of 12 phenols including the subtypes of bibenzyl, phenanthrene, and fluorenone, which was used to evaluate the quality of Dendrobium chrysotoxum. Through the full scan mode, new compounds were elucidated. The new compounds were quantified by carrying out the analysis of the ratio of the standard solution areas to new compound areas versus analyte concentration. The limit of detection (LOD) and limit of quantification (LOQ) for phenols were 0.5 microg/mL-1 microg/mL and 1 microg/mL-2 microg/mL, respectively. Average recoveries of phenols were ranged from 83.2% to 97.5%. Reproducibility represented by the RSD percentage was from 2.3% to 8.7%. The average content of the four analytes, erianin, chrysotobibenzyl, Confusarin, and moscatilin, were more than 200 mg/kg, and the content of bibenzyl compounds was found to be the highest in Dendrobium chrysotoxum. Among these bibenzyl compounds, erianin was determined as the typical chemical marker from Dendrobium chrysotoxum. The newly established UPLC with a miniature mass detector method was found to be an appropriate tool for the quality assessment of Dendrobium chrysotoxum.
Phenolic compounds from the stems of Flickingeria fimbriata.[Pubmed:28278646]
Nat Prod Res. 2017 Jul;31(13):1518-1522.
Chemical investigation of Flickingeria fimbriata (Bl.) Hawkes (Orchidaceae) resulted in the isolation and identification of one new dihydrophenanthrene, 1,2,5,6,7-pentamethoxy-9,10-dihydrophenanthrene (1), together with seven known dihydrophenanthrenes, erianthridin (2), coelonin (3), 4-methoxy-9,10-dihydrophenanthrene-1,2,7-triol (4), lusianthridin (5), ephemeranthol A (6), flavanthridin (7) and hircinol (8), four known phenanthrenes, epheranthol B (9), nudol (10), denthyrsinin (11) and Confusarin (12), and one known bibenzyl, batatasin III (13). The structure of the new compound was elucidated by spectroscopic analysis (HRMS, 1D and 2D NMR). All the compounds were isolated from F. fimbriata for the first time except for compounds 5 and 12, and compounds 1, 3, 4, 8, 10, 11 and 13 were obtained from this genus for the first time. Compounds 1-4 showed moderate cytotoxic activity against HepG2 cells.
Dendrobium protoplast co-culture promotes phytochemical assemblage in vitro.[Pubmed:27837285]
Protoplasma. 2017 Jul;254(4):1517-1528.
The present study is intended to analyze the occurrence of potent, low produce, naturally occurring stilbenes in protoplasts of wild species and hybrids of Dendrobium. The wild species selected for the study was Dendrobium ovatum, endemic to Western Ghats of India. Protoplasts were isolated from leaves and tepal tissues of all the species and were cultured purely to generate homofusants and cross-cultured to raise heterofusants. Phytochemical composition of protoplast culture with atypical and pure microcolonies was performed using mass spectrometry. Enzyme cocktail of 4% pectinase together with 2% cellulase displayed the highest competence for protoplast isolations. Maximum protoplast density of 30.11 x 10(4)/ml was obtained from D. ovatum leaves in 2 h. Subcellular features such as the presence of partially formed cell wall, the position of the nucleus, chloroplast density, colony existence, and integrity of the plasma membrane were analyzed. Among the pure and cross-cultured protoplasts, the number of heterofusants and homofusants formed were enumerated. The spectral feature extraction of the mass spectrometry indicated the presence of five phenolic marker compounds, viz., tristin, Confusarin, gigantol, moscatilin, and resveratrol, some of them in pure and others in assorted protoplast cultures raised from Dendrobium leaves and tepals. The study demonstrated that protoplast fusion technique enabled phytochemical assemblage in vitro as stilbenes tend to get restricted either in a tissue or species specific manner. This is the first report showing the presence of resveratrol, moscatilin, tristin, gigantol, and Confusarin in wild and hybrid species from cultured Dendrobium protoplasts in vitro.
Chemical composition, potential toxicity, and quality control procedures of the crude drug of Cyrtopodium macrobulbon.[Pubmed:24818583]
J Ethnopharmacol. 2014 Jul 3;154(3):790-7.
ETHNOPHARMACOLOGICAL RELEVANCE: Cyrtopodium macrobulbon ("canaveral") has been long used in Mexican traditional medicine for the treatment of painful urinary ailments ("mal de orin") in men. This study was conducted (i) to establish the potential acute toxicity and the antinociceptive activity of some preparations of Cyrtopodium macrobulbon, in order to demonstrate its preclinical efficacy for treating symptoms of "mal de orin"; and (ii) to determine the chemical composition and quality control parameters of this medicinal orchid. MATERIALS AND METHODS: The antinociceptive effect was assessed using the acetic acid-induced writhing and the hot-plate tests. Investigation of the acute toxicity was accomplished by the Lorke method. The organic extract (OE) was subjected to conventional phytochemical study using chromatographic conventional procedures. The volatile components profile of the species was accomplished via GC-MS analysis of HS-SPME-adsorbed compounds. Furthermore, an HPLC method to quantify ephemeranthol B (10) was developed and validated according to the International Conference on Harmonization Guidelines. Microscopic anatomy studies were performed using light and scanning electron microscopies. Finally, a potential distribution map was generated using the MaxEnt modeling method. RESULTS: AE and OE were not toxic to mice since the LD50 was higher than 5000 mg/kg. OE was only active in the acetic acid-induced writhing assay at the doses of 100 and 316 mg/kg. Conventional phytochemical analysis of OE led to the isolation and characterization of n-hexacosyl-trans-p-coumarate (1), n-octacosyl-trans-p-coumarate (2), n-triacontyl-trans-p-coumarate (3), 4-methoxy-benzyl alcohol (4), 4-hydroxybenzaldehyde (5), 1,5,7-trimethoxy-9,10-dihydrophenanthrene-2,6-diol (6), Confusarin (7), gigantol (8), batatasin III (9), and ephemeranthol B (10). The major volatile components identified by HS-SPME analysis were 6,10,14-trimethyl-2-pentadecanone, eucalyptol (11), and isobornyl formate. An HPLC analytical method for the quantification of compound 10 in the plant was developed and fully validated for selectivity, accuracy, and precision. The microscopic studies revealed that the epidermal tissue displayed a layer of enlarged, crenate and cell thin-walled cells with a thickened cuticle; these cells are described for first time for this species. The potential distribution map generated revealed that this species is widespread in Mexico from Sinaloa to Merida states. CONCLUSIONS: The results of the pharmacological studies tend to support the traditional use of Cyrtopodium macrobulbon for "mal de orin"; the presence of compounds 8, 9, and 11 with known antinociceptive activity might be related with the pharmacological effect demonstrated. The HPLC and microscopic analyses developed in this work will be valuable tools for quality control purposes for this plant.
[Studies on phenanthrene constituents from stems of Dendrobium candidum].[Pubmed:19504966]
Zhong Yao Cai. 2009 Feb;32(2):220-3.
OBJECTIVE: To investigate chemical constituents from the stems of Dendrobium candidum. METHODS: The constituents were isolated by various column chromatography methods, and their structures were elucidated by spectral analysis (IR, UV, NMR and MS). RESULTS: Six compounds were isolated from ethanol extract and their structures were identified as 2,3,4,7-tetramethoxyphenanthrene (I), nakaharain (II), 2,5-dihydroxy-3,4-dimethoxyphenanthrene (III), Confusarin (IV), nudol (V) and bulbophyllanthrin (VI). CONCLUSION: Among these compounds, compounds II, III, VI are isolated from Dendrobium candidum for the first time.
Cytotoxic phenanthrenes from the rhizomes of Tamus communis.[Pubmed:16783700]
Planta Med. 2006 Jun;72(8):767-70.
From the fresh rhizomes of Tamus communis five phenanthrenes (1 - 5) were isolated under the guidance of cytotoxic assays in HeLa cells. The compounds were obtained from the highly active CHCl (3) fraction of the MeOH extract by using multistep chromatographic purifications, including VLC, preparative TLC, HPLC and gel filtration. The compounds were identified by means of EI-mass, UV and NMR spectroscopy as 7-hydroxy-2,3,4-trimethoxyphenanthrene (1), 2,7-dihydroxy-3,4-dimethoxyphenanthrene (nudol) (2), 2,7-dihydroxy-3,4,8-trimethoxyphenanthrene (3), 3,7-dihydroxy-2,4,8-trimethoxyphenanthrene (Confusarin) (4), and 3,7-dihydroxy-2,4-dimethoxyphenanthrene (5). Compound 1 is a new natural product, and 2 - 4 were isolated for the first time from T. communis. In the cytotoxic assays, compounds 1 - 3 and 5 significantly inhibited the growth of HeLa cells (IC (50) = 0.97 - 20.18 microM). Compound 3, with an IC (50) value of 0.97 microM, is of special interest because of its high activity.
[Studies on the chemical constituents of Dendrobium fimbriatum].[Pubmed:14515799]
Yao Xue Xue Bao. 2003 Jul;38(7):526-9.
AIM: To investigate the chemical constituents of Dendrobium fimbriatum Hook. METHODS: Various chromatographic techniques were employed for isolation and purification of the constituents. The structures were elucidated by IR, MS, 1HNMR, 13CNMR and 2D-NMR. RESULTS: Eight compounds were obtained and identified by spectral analysis as fimbriatone, Confusarin, crepidatin, physcion, rhein, ayapin, scopolin methyl ether and n-octacostyl ferulate. CONCLUSION: Fimbriatone is a new compound, physcion and rhein are firstly isolated from Genus Dendrobium. The others are found from this species for the first time. Fimbriatone showed potential inhibitory effects on BGC cell line.


