DenthyrsininCAS# 118169-17-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 118169-17-8 | SDF | Download SDF |
| PubChem ID | 11779542.0 | Appearance | Powder |
| Formula | C17H16O5 | M.Wt | 300.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
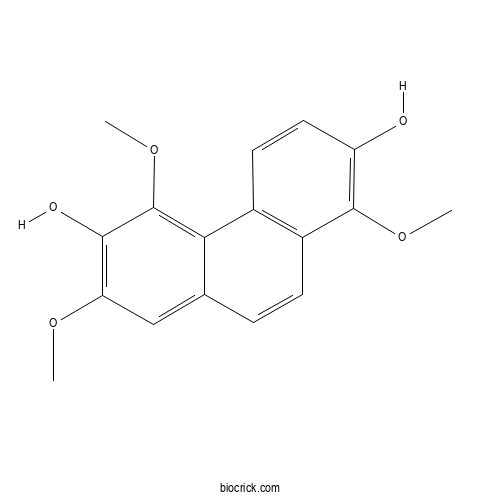
| Chemical Name | 1,5,7-trimethoxyphenanthrene-2,6-diol | ||
| SMILES | COC1=C(C(=C2C(=C1)C=CC3=C2C=CC(=C3OC)O)OC)O | ||
| Standard InChIKey | LLRLPRUFUGWYOL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H16O5/c1-20-13-8-9-4-5-11-10(6-7-12(18)16(11)21-2)14(9)17(22-3)15(13)19/h4-8,18-19H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Denthyrsinin Dilution Calculator

Denthyrsinin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3299 mL | 16.6495 mL | 33.2989 mL | 66.5978 mL | 83.2473 mL |
| 5 mM | 0.666 mL | 3.3299 mL | 6.6598 mL | 13.3196 mL | 16.6495 mL |
| 10 mM | 0.333 mL | 1.6649 mL | 3.3299 mL | 6.6598 mL | 8.3247 mL |
| 50 mM | 0.0666 mL | 0.333 mL | 0.666 mL | 1.332 mL | 1.6649 mL |
| 100 mM | 0.0333 mL | 0.1665 mL | 0.333 mL | 0.666 mL | 0.8325 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9-epi-6-Methoxygeniposidic acid
Catalog No.:BCX1496
CAS No.:1215178-87-2
- 12β-Acetoxycoccinic acid
Catalog No.:BCX1495
CAS No.:125247-74-7
- 11-O-Syringylbergenin
Catalog No.:BCX1494
CAS No.:126485-47-0
- 7,4'-Di-O-methyltectorigenin
Catalog No.:BCX1493
CAS No.:13186-08-8
- Ginsenoside CY
Catalog No.:BCX1492
CAS No.:83480-65-3
- ent-kauran-16β,17-diol
Catalog No.:BCX1491
CAS No.:16836-31-0
- Erucic acid
Catalog No.:BCX1490
CAS No.:112-86-7
- Dihydrohelenalin
Catalog No.:BCX1489
CAS No.:34257-95-9
- 12-Hydroxyganoderenic acid B
Catalog No.:BCX1488
CAS No.:1309931-84-7
- Paroxetine Impurity A Hydrochloride salt
Catalog No.:BCX1487
CAS No.:1394842-91-1
- Fluvoxamine Impurity D
Catalog No.:BCX1486
CAS No.:61718-80-7
- Aceclofenac impurity I
Catalog No.:BCX1485
CAS No.:15362-40-0
- Confusarin
Catalog No.:BCX1498
CAS No.:108909-02-0
- Swertiaside
Catalog No.:BCX1499
CAS No.:96087-14-8
- Potentillanoside A
Catalog No.:BCX1500
CAS No.:1309589-79-4
- Homocapsaicin II
Catalog No.:BCX1501
CAS No.:71240-51-2
- Regaloside E
Catalog No.:BCX1502
CAS No.:123134-21-4
- Hydroxylinderstrenolide
Catalog No.:BCX1503
CAS No.:20267-92-9
- Visamminol-3'-O-glucoside
Catalog No.:BCX1504
CAS No.:2254096-98-3
- Tetrahydrodehydrodiconiferyl alcohol
Catalog No.:BCX1505
CAS No.:5234-70-8
- Fulvotomentoside A
Catalog No.:BCX1506
CAS No.:150107-44-1
- Sarsasapogenin acetate
Catalog No.:BCX1507
CAS No.:35319-91-6
- 4'-Hydroxyflavone
Catalog No.:BCX1508
CAS No.:4143-63-9
- Isoemetine hydrobromide
Catalog No.:BCX1509
CAS No.:21026-77-7
Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis.[Pubmed:34371579]
Plants (Basel). 2021 Jul 5;10(7):1376.
The Dendrobium species (Orchidaceae) has been cultivated as an ornamental plant as well as used in traditional medicines. In this study, the chemical profiles of Dendrobii Herba, used as herbal medicine, Dendrobium in two different species, their hybrid, and the gamma-irradiated mutant lines of the hybrid, were systematically investigated via ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-QToF MS). Among the numerous peaks detected, 17 peaks were unambiguously identified. Gigantol (1), (1R,2R)-1,7-hydroxy-2,8-methoxy-2,3-dihydrophenanthrene-4(1H)-one (2), tristin (3), (-)-syringaresinol (4), lusianthridin (5), 2,7-dihydroxy-phenanthrene-1,4-dione (6), densiflorol B (7), Denthyrsinin (8), moscatilin (9), lusianthridin dimer (10), batatasin III (11), ephemeranthol A (12), thunalbene (13), dehydroorchinol (14), dendrobine (15), shihunine (16), and 1,5,7-trimethoxy-2-phenanthrenol (17), were detected in Dendrobii Herba, while 1, 2, and 16 were detected in D. candidum, 1, 11, and 16 in D. nobile, and 1, 2, and 16 in the hybrid, D. nobile x candidum. The methanol extract taken of them was also examined for cytotoxicity against FaDu human hypopharynx squamous carcinoma cells, where Dendrobii Herba showed the greatest cytotoxicity. In the untargeted metabolite analysis of 436 mutant lines of the hybrid, using UPLC-QToF MS and cytotoxicity measurements combined with multivariate analysis, two tentative flavonoids (M1 and M2) were evaluated as key markers among the analyzed metabolites, contributing to the distinction between active and inactive mutant lines.
Phenolic compounds from the stems of Flickingeria fimbriata.[Pubmed:28278646]
Nat Prod Res. 2017 Jul;31(13):1518-1522.
Chemical investigation of Flickingeria fimbriata (Bl.) Hawkes (Orchidaceae) resulted in the isolation and identification of one new dihydrophenanthrene, 1,2,5,6,7-pentamethoxy-9,10-dihydrophenanthrene (1), together with seven known dihydrophenanthrenes, erianthridin (2), coelonin (3), 4-methoxy-9,10-dihydrophenanthrene-1,2,7-triol (4), lusianthridin (5), ephemeranthol A (6), flavanthridin (7) and hircinol (8), four known phenanthrenes, epheranthol B (9), nudol (10), Denthyrsinin (11) and confusarin (12), and one known bibenzyl, batatasin III (13). The structure of the new compound was elucidated by spectroscopic analysis (HRMS, 1D and 2D NMR). All the compounds were isolated from F. fimbriata for the first time except for compounds 5 and 12, and compounds 1, 3, 4, 8, 10, 11 and 13 were obtained from this genus for the first time. Compounds 1-4 showed moderate cytotoxic activity against HepG2 cells.
Bi-bicyclic and bi-tricyclic compounds from Dendrobium thyrsiflorum.[Pubmed:15913675]
Phytochemistry. 2005 May;66(10):1113-20.
One bi-bicyclic and two bi-tricyclic derivatives of coumarin-benzofuran, phenanthrene-phenanthrene and phenanthrene-phenanthraquinone, along with seven known compounds, were isolated from stems of Dendrobium thyrsiflorum Rchb.f. (Orchidaceae). On the basis of chemical, NMR (1H, 13C, HMQC, HMBC and NOESY) and mass spectrometry data, their structures were elucidated as denthyrsin [3-(5',6'-dimethoxybenzofuran-2'-yl)-6,7-dimethoxy-2H-chromen-2-one; 1], denthyrsinol (4,5'-dimethoxy-[1,1']biphenanthrenyl-2,5,4',7'-tetraol; 2), and denthyrsinone (7,4',7'-trihydroxy-2,2',8'-trimethoxy-[5,1']biphenanthrenyl-1,4-dione; 3). Compounds 1-3 and Denthyrsinin (1,5,7-trimethoxyphenanthrene-2,6-diol; 4) showed significant cytotoxic activities against Hela (13.5, 9.3, 9.9 and 2.7 microM, respectively), K-562 (0.45, 1.6, 6.0 and 2.3 microM, respectively) and MCF-7 (18.1, not tested, 3.5 and 4.8 microM, respectively) cell lines.


