DihydrohelenalinCAS# 34257-95-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34257-95-9 | SDF | Download SDF |
| PubChem ID | 4364540.0 | Appearance | Powder |
| Formula | C15H20O4 | M.Wt | 264.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 9-hydroxy-1,5,8a-trimethyl-3a,4,5,5a,9,9a-hexahydro-1H-azuleno[6,7-b]furan-2,8-dione | ||
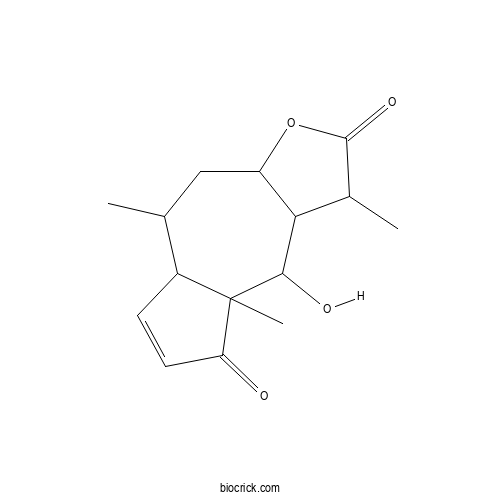
| SMILES | CC1CC2C(C(C(=O)O2)C)C(C3(C1C=CC3=O)C)O | ||
| Standard InChIKey | ICKWITMQEROMDG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20O4/c1-7-6-10-12(8(2)14(18)19-10)13(17)15(3)9(7)4-5-11(15)16/h4-5,7-10,12-13,17H,6H2,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dihydrohelenalin Dilution Calculator

Dihydrohelenalin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7833 mL | 18.9165 mL | 37.8329 mL | 75.6659 mL | 94.5823 mL |
| 5 mM | 0.7567 mL | 3.7833 mL | 7.5666 mL | 15.1332 mL | 18.9165 mL |
| 10 mM | 0.3783 mL | 1.8916 mL | 3.7833 mL | 7.5666 mL | 9.4582 mL |
| 50 mM | 0.0757 mL | 0.3783 mL | 0.7567 mL | 1.5133 mL | 1.8916 mL |
| 100 mM | 0.0378 mL | 0.1892 mL | 0.3783 mL | 0.7567 mL | 0.9458 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 12-Hydroxyganoderenic acid B
Catalog No.:BCX1488
CAS No.:1309931-84-7
- Paroxetine Impurity A Hydrochloride salt
Catalog No.:BCX1487
CAS No.:1394842-91-1
- Fluvoxamine Impurity D
Catalog No.:BCX1486
CAS No.:61718-80-7
- Aceclofenac impurity I
Catalog No.:BCX1485
CAS No.:15362-40-0
- Monbarbatain A
Catalog No.:BCX1484
CAS No.:138711-55-4
- Gymconopin C
Catalog No.:BCX1483
CAS No.:844493-85-2
- (±)-Taxifolin
Catalog No.:BCX1482
CAS No.:24198-97-8
- Purpurogallin carboxylic acid
Catalog No.:BCX1481
CAS No.:5146-12-3
- Ganoderic acid Epsilon
Catalog No.:BCX1480
CAS No.:294674-05-8
- 20(S)-25-Hydroxyprotopanaxadiol
Catalog No.:BCX1479
CAS No.:66007-91-8
- 20(S)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1478
CAS No.:66007-93-0
- 20(S)-25-Hydroxyprotopanaxatiol
Catalog No.:BCX1477
CAS No.:113566-83-9
- Erucic acid
Catalog No.:BCX1490
CAS No.:112-86-7
- ent-kauran-16β,17-diol
Catalog No.:BCX1491
CAS No.:16836-31-0
- Ginsenoside CY
Catalog No.:BCX1492
CAS No.:83480-65-3
- 7,4'-Di-O-methyltectorigenin
Catalog No.:BCX1493
CAS No.:13186-08-8
- 11-O-Syringylbergenin
Catalog No.:BCX1494
CAS No.:126485-47-0
- 12β-Acetoxycoccinic acid
Catalog No.:BCX1495
CAS No.:125247-74-7
- 9-epi-6-Methoxygeniposidic acid
Catalog No.:BCX1496
CAS No.:1215178-87-2
- Denthyrsinin
Catalog No.:BCX1497
CAS No.:118169-17-8
- Confusarin
Catalog No.:BCX1498
CAS No.:108909-02-0
- Swertiaside
Catalog No.:BCX1499
CAS No.:96087-14-8
- Potentillanoside A
Catalog No.:BCX1500
CAS No.:1309589-79-4
- Homocapsaicin II
Catalog No.:BCX1501
CAS No.:71240-51-2
Influence of Abiotic and Biotic Elicitors on Organogenesis, Biomass Accumulation, and Production of Key Secondary Metabolites in Asteraceae Plants.[Pubmed:38673783]
Int J Mol Sci. 2024 Apr 10;25(8):4197.
The medicinal plants of the Asteraceae family are a valuable source of bioactive secondary metabolites, including polyphenols, phenolic acids, flavonoids, acetylenes, sesquiterpene lactones, triterpenes, etc. Under stressful conditions, the plants develop these secondary substances to carry out physiological tasks in plant cells. Secondary Asteraceae metabolites that are of the greatest interest to consumers are artemisinin (an anti-malarial drug from Artemisia annua L.-sweet wormwood), steviol glycosides (an intense sweetener from Stevia rebaudiana Bert.-stevia), caffeic acid derivatives (with a broad spectrum of biological activities synthesized from Echinacea purpurea (L.) Moench-echinacea and Cichorium intybus L.-chicory), helenalin and Dihydrohelenalin (anti-inflammatory drug from Arnica montana L.-mountain arnica), parthenolide ("medieval aspirin" from Tanacetum parthenium (L.) Sch.Bip.-feverfew), and silymarin (liver-protective medicine from Silybum marianum (L.) Gaertn.-milk thistle). The necessity to enhance secondary metabolite synthesis has arisen due to the widespread use of these metabolites in numerous industrial sectors. Elicitation is an effective strategy to enhance the production of secondary metabolites in in vitro cultures. Suitable technological platforms for the production of phytochemicals are cell suspension, shoots, and hairy root cultures. Numerous reports describe an enhanced accumulation of desired metabolites after the application of various abiotic and biotic elicitors. Elicitors induce transcriptional changes in biosynthetic genes, leading to the metabolic reprogramming of secondary metabolism and clarifying the mechanism of the synthesis of bioactive compounds. This review summarizes biotechnological investigations concerning the biosynthesis of medicinally essential metabolites in plants of the Asteraceae family after various elicitor treatments.
Arnica montana L.: Doesn't Origin Matter?[Pubmed:37895999]
Plants (Basel). 2023 Oct 11;12(20):3532.
Arnica montana L. (Asteraceae) has a long and successful tradition in Europe as herbal medicine. Arnica flowers (i.e., the flowerheads of Arnica montana) are monographed in the European Pharmacopoeia (Ph. Eur.), and a European Union herbal monograph exists, in which its use as traditional herbal medicine is recommended. According to this monograph, Arnica flowers (Arnicae flos Ph. Eur.) and preparations thereof may be used topically to treat blunt injuries and traumas, inflammations and rheumatic muscle and joint complaints. The main bioactive constituents are sesquiterpene lactones (STLs) of the helenanolide type. Among these, a variety of esters of helenalin and 11alpha,13-Dihydrohelenalin with low-molecular-weight carboxylic acids, namely, acetic, isobutyric, methacrylic, methylbutyric as well as tiglic acid, represent the main constituents, in addition to small amounts of the unesterified parent STLs. A plethora of reports exist on the pharmacological activities of these STLs, and it appears unquestioned that they represent the main active principles responsible for the herbal drug's efficacy. It has been known for a long time, however, that considerable differences in the STL pattern occur between A. montana flowers from plants growing in middle or Eastern Europe with some originating from the Iberic peninsula. In the former, Helenalin esters usually predominate, whereas the latter contains almost exclusively 11alpha,13-Dihydrohelenalin derivatives. Differences in pharmacological potency, on the other hand, have been reported for the two subtypes of Arnica-STLs in various instances. At the same time, it has been previously proposed that one should distinguish between two subspecies of A. montana, subsp. montana occurring mainly in Central and Eastern Europe and subsp. atlantica in the southwestern range of the species distribution, i.e., on the Iberian Peninsula. The question hence arises whether or not the geographic origin of Arnica montana flowers is of any relevance for the medicinal use of the herbal drug and the pharmaceutical quality, efficacy and safety of its products and whether the chemical/pharmacological differences should not be recognized in pharmacopoeia monographs. The present review attempts to answer these questions based on a summary of the current state of botanical, phytochemical and pharmacological evidence.
Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa.[Pubmed:37298857]
Molecules. 2023 May 27;28(11):4379.
Arnica montana is a valuable plant with high demand on the pharmaceutical and cosmetic market due to the presence of helenalin (H) and 11alpha, 13-Dihydrohelenalin (DH) sesquiterpene lactones (SLs), with many applications and anti-inflammatory, anti-tumor, analgesic and other properties. Despite the great importance of these compounds for the protection of the plant and their medicinal value, the content of these lactones and the profile of the compounds present within individual elements of florets and flower heads have not been studied so far, and attempts to localize these compounds in flower tissues have also not been conducted. The three studied Arnica taxa synthesize SLs only in the aerial parts of plants, and the highest content of these substances was found in A. montana cv. Arbo; it was lower in wild species, and a very small amount of H was produced by A. chamissonis. Analysis of dissected fragments of whole inflorescences revealed a specific distribution pattern of these compounds. The lactones content in single florets increased from the top of the corolla to the ovary, with the pappus calyx being a significant source of their production. Histochemical tests for terpenes and methylene ketones indicated the colocalization of lactones with inulin vacuoles.
Dermal Absorption of Sesquiterpene Lactones from Arnica Tincture.[Pubmed:35456576]
Pharmaceutics. 2022 Mar 29;14(4):742.
Arnica tincture is a traditional herbal medicine used to treat blunt injuries, e.g., bruises and squeezes. In addition, a potential new use in the treatment of cutaneous leishmaniasis is currently under investigation. Therefore, detailed information about the dermal absorption of the tincture and especially its bioactive constituents, sesquiterpene lactones (STLs) of the helenalin- and 11alpha,13-Dihydrohelenalin type, is mandatory. Consequently, this article reports on dermal absorption studies of Arnica tincture using diffusion cells and porcine skin as well as two human skin samples with different permeability. The amounts of STLs on the skin surfaces, in skin extracts and in the receptor fluids were quantified by ultra-high-performance liquid chromatography with high-resolution mass spectrometry (UHPLC-HRMS). It was found that Arnica STLs permeated into the receptor fluid already 4 h after the application, but the amount was rather low. Within 48 h, a maximum of 8.4%, 14.6% and 36.4% of STLs permeated through porcine skin, human skin A (trans-epidermal water loss (TEWL) = 11.518 g.m(-2).h(-1)) and the more permeable human skin B (TEWL = 17.271 g.m(-2).h(-1)), respectively. The majority of STLs was absorbed (penetrated into the skin; 97.6%, 97.8% and 99.3%) after 48 h but a huge portion could not be extracted from skin and is expected to be irreversibly bound to skin proteins. To better visualize the analytes in different skin layers, a fluorescence-labeled STL, helenalin 3,4-dimethoxycinnamate, was synthesized. Fluorescence microscopic images depict an accumulation of the fluorescent derivative in the epidermis. For the treatment of local, cutaneous complaints, an enrichment of the bioactive substances in the skin may be considered beneficial.
Soil and Vegetation Drive Sesquiterpene Lactone Content and Profile in Arnica montana L. Flower Heads From Apuseni-Mountains, Romania.[Pubmed:35154225]
Front Plant Sci. 2022 Jan 28;13:813939.
Arnica montana L. (AM, Asteraceae) is a perennial, herbaceous vascular plant species of commercial importance. The flower heads' pharmacological properties are attributed mainly to sesquiterpene lactones (SLs), with phenolic acids and flavonoids also considered of relevance. The botanical drug is still partly collected in different European mountain regions. The SL content can be influenced by genetic factors and environmental conditions (altitude, temperature and rainfall). Surprisingly, the influence of the soil on SL-content have rarely been investigated. However, the soil determines the occurrence, distribution and overall fitness of AM. Equally, environmental factors are crucial determinants for the biosynthesis and fluctuations in plant secondary metabolites. Therefore, different abiotic (pH, C/N ratio, base saturation, cation exchange capacity) and biotic (species richness, vegetation cover) parameters need to be assessed as potential drivers of the variable content of AM's secondary metabolites. Consequently, we developed an in situ experimental design aiming to cover a wide range of soil pH conditions. We detected and investigated different AM populations growing in grassland on acidic soils, on siliceous as well as calcareous geologies within the same geographical region and altitudinal belt. The total SL content and most single SL contents of the AM flower heads differed significantly between the two geologies. AM flower heads of plants growing on loam on limestone showed a significant higher total SL content than the flower heads of plants growing in siliceous grasslands. Furthermore, the SL contents were significantly correlated with geobotanical species richness and vegetation cover pointing toward an effect of species interactions on the production of SLs. Moreover, the ratios of the main SLs helenalin to Dihydrohelenalin esters were significantly correlated to environmental parameters indicating that SL composition might be a function of habitat conditions. The findings of this study shed light upon the often ignored, complex interactions between environmental conditions and plant secondary metabolites. We highlight the importance of both abiotic and biotic habitat parameters for SLs in AM.
In Vitro Metabolism of Helenalin Acetate and 11alpha,13-Dihydrohelenalin Acetate: Natural Sesquiterpene Lactones from Arnica.[Pubmed:35050210]
Metabolites. 2022 Jan 17;12(1):88.
Arnica tincture is a herbal medicinal preparation with anti-inflammatory activity which is used traditionally for the topical treatment of blunt injuries as well as rheumatic muscle and joint complaints. Its main bioactive constituents are sesquiterpene lactones (STLs) of the helenalin and 11alpha,13-Dihydrohelenalin types. Besides the mentioned activity, the tincture and its isolated STLs have antileishmanial activity. In a recent in vivo study, a treatment with Arnica tincture cured cutaneous Leishmaniasis (CL) in a golden hamster model. CL is a neglected tropical disease affecting more than two million people every year, for which new treatments are urgently needed. In order to use Arnica tincture on open CL lesions of human patients, it is important to know how the constituents are metabolized. Therefore, in vitro metabolism experiments with liver microsomes of different species (rat, pig and human) were performed with the Arnica STLs helenalin acetate and 11alpha,13-Dihydrohelenalin acetate. Phase I and phase II metabolism experiments were performed, as well as a combination of both. Glutathione conjugation plays a major role in the metabolism of these STLs, as could be expected based on previous reports on their reactivity. Besides glutathione conjugates, several other metabolites were formed, e.g., water conjugates and hydroxides. Our results show for the first time a detailed picture of the metabolism of Arnica STLs. The fast and extensive formation of glutathione conjugates makes it unlikely that low absorbed levels of these compounds, as expected after dermal absorption from Arnica tincture, could be of toxicological concern.


