Ganoderic acid EpsilonCAS# 294674-05-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 294674-05-8 | SDF | Download SDF |
| PubChem ID | 15427810.0 | Appearance | Powder |
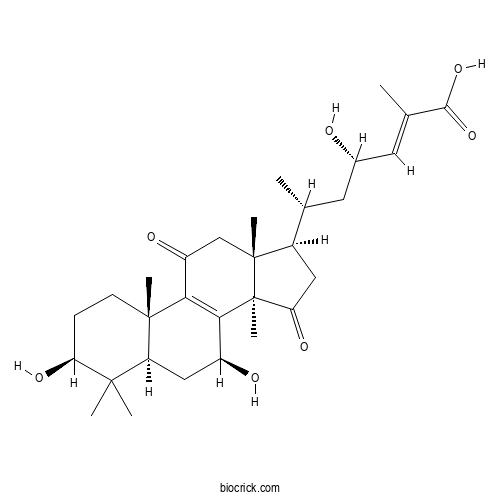
| Formula | C30H44O7 | M.Wt | 516.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ganoderic acid ε | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E,4S,6R)-6-[(3S,5R,7S,10S,13R,14R,17R)-3,7-dihydroxy-4,4,10,13,14-pentamethyl-11,15-dioxo-2,3,5,6,7,12,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-4-hydroxy-2-methylhept-2-enoic acid | ||
| SMILES | CC(CC(C=C(C)C(=O)O)O)C1CC(=O)C2(C1(CC(=O)C3=C2C(CC4C3(CCC(C4(C)C)O)C)O)C)C | ||
| Standard InChIKey | MIWGXXQCEDNROQ-UZOYOLKESA-N | ||
| Standard InChI | InChI=1S/C30H44O7/c1-15(10-17(31)11-16(2)26(36)37)18-12-23(35)30(7)25-19(32)13-21-27(3,4)22(34)8-9-28(21,5)24(25)20(33)14-29(18,30)6/h11,15,17-19,21-22,31-32,34H,8-10,12-14H2,1-7H3,(H,36,37)/b16-11+/t15-,17+,18-,19+,21+,22+,28+,29-,30+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ganoderic acid Epsilon Dilution Calculator

Ganoderic acid Epsilon Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9355 mL | 9.6774 mL | 19.3547 mL | 38.7094 mL | 48.3868 mL |
| 5 mM | 0.3871 mL | 1.9355 mL | 3.8709 mL | 7.7419 mL | 9.6774 mL |
| 10 mM | 0.1935 mL | 0.9677 mL | 1.9355 mL | 3.8709 mL | 4.8387 mL |
| 50 mM | 0.0387 mL | 0.1935 mL | 0.3871 mL | 0.7742 mL | 0.9677 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1935 mL | 0.3871 mL | 0.4839 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 20(S)-25-Hydroxyprotopanaxadiol
Catalog No.:BCX1479
CAS No.:66007-91-8
- 20(S)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1478
CAS No.:66007-93-0
- 20(S)-25-Hydroxyprotopanaxatiol
Catalog No.:BCX1477
CAS No.:113566-83-9
- (20S)-Panaxadiol
Catalog No.:BCX1476
CAS No.:112791-34-1
- (20S)-Panaxatriol
Catalog No.:BCX1475
CAS No.:848830-68-2
- Oleuropein Aglycone
Catalog No.:BCX1474
CAS No.:31773-95-2
- Ganoderic acid Gama
Catalog No.:BCX1473
CAS No.:294674-00-3
- Ganoderenic acid C2
Catalog No.:BCX1472
CAS No.:1961358-00-8
- Iodixanol Impurity E
Catalog No.:BCX1471
CAS No.:255376-57-9
- Salicortin
Catalog No.:BCX1470
CAS No.:1887055-63-1
- 4-Hydroxylonchocarpin
Catalog No.:BCX1469
CAS No.:56083-03-5
- Dehydroandrographolide Succinate Sodium Potasium Salt
Catalog No.:BCX1468
CAS No.:863319-40-8
- Purpurogallin carboxylic acid
Catalog No.:BCX1481
CAS No.:5146-12-3
- (±)-Taxifolin
Catalog No.:BCX1482
CAS No.:24198-97-8
- Gymconopin C
Catalog No.:BCX1483
CAS No.:844493-85-2
- Monbarbatain A
Catalog No.:BCX1484
CAS No.:138711-55-4
- Aceclofenac impurity I
Catalog No.:BCX1485
CAS No.:15362-40-0
- Fluvoxamine Impurity D
Catalog No.:BCX1486
CAS No.:61718-80-7
- Paroxetine Impurity A Hydrochloride salt
Catalog No.:BCX1487
CAS No.:1394842-91-1
- 12-Hydroxyganoderenic acid B
Catalog No.:BCX1488
CAS No.:1309931-84-7
- Dihydrohelenalin
Catalog No.:BCX1489
CAS No.:34257-95-9
- Erucic acid
Catalog No.:BCX1490
CAS No.:112-86-7
- ent-kauran-16β,17-diol
Catalog No.:BCX1491
CAS No.:16836-31-0
- Ginsenoside CY
Catalog No.:BCX1492
CAS No.:83480-65-3
A new triterpene from the fruiting bodies of Ganoderma lucidum.[Pubmed:19806918]
Yao Xue Xue Bao. 2009 Jul;44(7):768-70.
A new lanostanoid triterpene, named ganoderitriol M (1), together with a known triterpene Ganoderic acid Epsilon (2), were isolated from the fruiting bodies of G lucidum. Compound 1 was deduced as (24S)-lanosta-7-oxo-8-en-3beta, 24, 25-triol on the basis of spectral analysis (UV, IR, MS, 1H NMR, 13C NMR and 2D NMR).
A new lanostane-type triterpene from the fruiting bodies of Ganoderma lucidum.[Pubmed:12067158]
J Asian Nat Prod Res. 2002 Jun;4(2):129-34.
A new lanostane-type triterpene, named ganoderic acid LM2 (5), was isolated from the fruiting bodies of Ganoderma lucidum. Its structure was characterized as (23S) 7beta, -dihydroxy-3, 11, 15-trioxo-5alpha-lanosta-8, 24-dien-26-oic acid by 1D- and 2D-NMR spectra. In addition, a known triterpene, Ganoderic acid Epsilon (4), was obtained. Both of them exhibited potent enhancement of ConA-induced mice splenocytes proliferation in vitro.
Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells.[Pubmed:10923835]
Chem Pharm Bull (Tokyo). 2000 Jul;48(7):1026-33.
Six new highly oxygenated lanostane-type triterpenes, called ganoderic acid gamma (1), ganoderic acid delta (2), Ganoderic acid Epsilon (3), ganoderic acid zeta (4), ganoderic acid eta (5) and ganoderic acid theta (6), were isolated from the spores of Ganoderma lucidum, together with known ganolucidic acid D (7) and ganoderic acid C2 (8). Their structures of the new triterpenes were determined as (23S)-7beta,15alpha,23-trihydroxy-3,11-dioxolanosta-8, 24(E)-diene-26-oic acid (1), (23S)-7alpha,15alpha23-trihydroxy-3,11-dioxolanosta-8, 24(E)-diene-26-oic acid (2), (23S)-3beta3,7beta, 23-trihydroxy-11,15-dioxolanosta-8,24(E)-diene-26-oic acid (3), (23S)-3beta,23-dihydroxy-7,11,15-trioxolanosta-8, 24(E)-diene-26-oic acid (4), (23S)-3beta,7beta,12beta,23-tetrahydroxy-11,15-dioxolanos ta-8,24(E)-diene-26-oic acid (5) and (23S)-3beta,12beta23-trihydroxy-7,11,15-trioxolanosta-8,24(E )-diene-26-oic acid (6), respectively, by chemical and spectroscopic means, which included the determination of a chiral center in the side chain by a modification of Mosher's method. The cytotoxicity of the compounds isolated from the Ganoderma spores was carried out in vitro against Meth-A and LLC tumor cell lines.


