Monbarbatain ACAS# 138711-55-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 138711-55-4 | SDF | Download SDF |
| PubChem ID | 85625420.0 | Appearance | Powder |
| Formula | C30H22O6 | M.Wt | 478.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
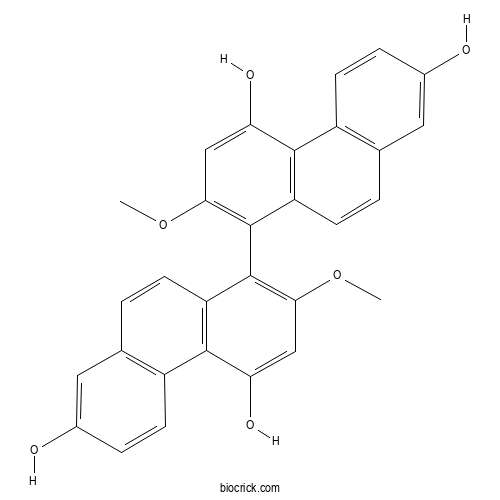
| Chemical Name | 8-(4,7-dihydroxy-2-methoxyphenanthren-1-yl)-7-methoxyphenanthrene-2,5-diol | ||
| SMILES | COC1=C(C2=C(C3=C(C=C2)C=C(C=C3)O)C(=C1)O)C4=C(C=C(C5=C4C=CC6=C5C=CC(=C6)O)O)OC | ||
| Standard InChIKey | RBVCTUJCRSUBKB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C30H22O6/c1-35-25-13-23(33)27-19-9-5-17(31)11-15(19)3-7-21(27)29(25)30-22-8-4-16-12-18(32)6-10-20(16)28(22)24(34)14-26(30)36-2/h3-14,31-34H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Monbarbatain A Dilution Calculator

Monbarbatain A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0899 mL | 10.4493 mL | 20.8986 mL | 41.7973 mL | 52.2466 mL |
| 5 mM | 0.418 mL | 2.0899 mL | 4.1797 mL | 8.3595 mL | 10.4493 mL |
| 10 mM | 0.209 mL | 1.0449 mL | 2.0899 mL | 4.1797 mL | 5.2247 mL |
| 50 mM | 0.0418 mL | 0.209 mL | 0.418 mL | 0.8359 mL | 1.0449 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.209 mL | 0.418 mL | 0.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gymconopin C
Catalog No.:BCX1483
CAS No.:844493-85-2
- (±)-Taxifolin
Catalog No.:BCX1482
CAS No.:24198-97-8
- Purpurogallin carboxylic acid
Catalog No.:BCX1481
CAS No.:5146-12-3
- Ganoderic acid Epsilon
Catalog No.:BCX1480
CAS No.:294674-05-8
- 20(S)-25-Hydroxyprotopanaxadiol
Catalog No.:BCX1479
CAS No.:66007-91-8
- 20(S)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1478
CAS No.:66007-93-0
- 20(S)-25-Hydroxyprotopanaxatiol
Catalog No.:BCX1477
CAS No.:113566-83-9
- (20S)-Panaxadiol
Catalog No.:BCX1476
CAS No.:112791-34-1
- (20S)-Panaxatriol
Catalog No.:BCX1475
CAS No.:848830-68-2
- Oleuropein Aglycone
Catalog No.:BCX1474
CAS No.:31773-95-2
- Ganoderic acid Gama
Catalog No.:BCX1473
CAS No.:294674-00-3
- Ganoderenic acid C2
Catalog No.:BCX1472
CAS No.:1961358-00-8
- Aceclofenac impurity I
Catalog No.:BCX1485
CAS No.:15362-40-0
- Fluvoxamine Impurity D
Catalog No.:BCX1486
CAS No.:61718-80-7
- Paroxetine Impurity A Hydrochloride salt
Catalog No.:BCX1487
CAS No.:1394842-91-1
- 12-Hydroxyganoderenic acid B
Catalog No.:BCX1488
CAS No.:1309931-84-7
- Dihydrohelenalin
Catalog No.:BCX1489
CAS No.:34257-95-9
- Erucic acid
Catalog No.:BCX1490
CAS No.:112-86-7
- ent-kauran-16β,17-diol
Catalog No.:BCX1491
CAS No.:16836-31-0
- Ginsenoside CY
Catalog No.:BCX1492
CAS No.:83480-65-3
- 7,4'-Di-O-methyltectorigenin
Catalog No.:BCX1493
CAS No.:13186-08-8
- 11-O-Syringylbergenin
Catalog No.:BCX1494
CAS No.:126485-47-0
- 12β-Acetoxycoccinic acid
Catalog No.:BCX1495
CAS No.:125247-74-7
- 9-epi-6-Methoxygeniposidic acid
Catalog No.:BCX1496
CAS No.:1215178-87-2
[Chemical constituents from Pleione bulbocodioides].[Pubmed:24946545]
Zhongguo Zhong Yao Za Zhi. 2014 Feb;39(3):442-7.
Fourteen compoumds were isolated from the ethyl acetate portion of the 95% ethanolic extract of Pleione bulbocodioides by a combination of various chromatographic techniques including silica gel, ODS, macroporous adsorbent resin, Sephadex LH-20, and preparative HPLC, of which ten compoumds were phenanthrenes and dihydrophenanthrenes, two compoumds were bibenzyls, one was lignan and a sterol. Their structures were identified on the basis of spectroscopic data as Monbarbatain A(1), 2, 7, 2'-trihy-droxy-4, 4', 7'-trimethoxy-1, 1'- biphenanthrene(2), blestriarene A(3), pleionesin B(4), shanciol H(5), 17-hydroxy-7'-(4'-hy-droxy-3 '-methoxyphenyl)- 4-methoxy-9, 10, 7', 8'-tetrahydrophenanthro[2, 3-b]furan-8'-yl methyl acetate(6), 1-p-hydroxybenzyl-4-methoxy phenanthrene-2, 7-diol(7), 1-p-hydroxybenzyl-4-met-hoxy-9, 10-dihydrophenanthrene-2, 7-diol(8), hircinol(9), coelonin( 10), gigantol(11), batatasin 11 (12), syringaresinol(13) and ergosta4, 6, 8 ( 14) , 22-tetraen-3-one (14). Compounds 1-3, 9, 13 and 14 were isolated from this genus for the first time.


