Cyclo(Phe-Val)CAS# 14474-71-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14474-71-6 | SDF | Download SDF |
| PubChem ID | 139766 | Appearance | Powder |
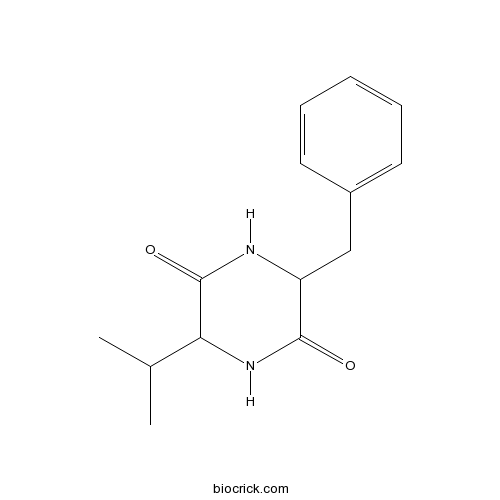
| Formula | C14H18N2O2 | M.Wt | 246.31 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-benzyl-6-propan-2-ylpiperazine-2,5-dione | ||
| SMILES | CC(C)C1C(=O)NC(C(=O)N1)CC2=CC=CC=C2 | ||
| Standard InChIKey | OQQPOHUVAQPSHJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H18N2O2/c1-9(2)12-14(18)15-11(13(17)16-12)8-10-6-4-3-5-7-10/h3-7,9,11-12H,8H2,1-2H3,(H,15,18)(H,16,17) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cyclo(Phe-Val) is a new cell cycle inhibitor, it shows cyctoxic activity in vitro. |
| In vitro | Cyclic dipeptide constituents from the mangrove fungus Penicillium oxalicum[Reference: WebLink]Journal of Shenyang Pharmaceutical University, 2007, 24(8):474-8.To study the metabolites of mangrove fungus Penicillium oxalicum from the south China sea and search for new anti-tumor compounds. Cyclic dipeptides as new cell cycle inhibitors produced by Streptomyces flavoretus 18522[Reference: WebLink]Journal of Shenyang Pharmaceutical University, 2015 (2):107 -10.To find the cell cycle inhibitors from the metabolites of Streptomyces flavoretus 18522. |
| Structure Identification | Evidence-Based Complementary and Alternative Medicine Volume 2011 (2011), Article ID 393752, 6 pagesDescription of a Sulfitobacter Strain and Its Extracellular Cyclodipeptides[Reference: WebLink]A marine bacterium M44 was separated from 30 m deep seawater in the East China Sea (26° 28.3′ N 122° 29.0′ E) in 2006. 16S rDNA gene sequence comparison showed that the strain M44 was a member of the genus Sulfitobacter and highly similar to KMM 3554T. A series of experiments demonstrated that this strain M44 had many distinctive characteristics: its cells were gram-negative and mesophilic; its colonies were slightly yellowish, round, convex, and smooth; and it could grow at 10–28°C, pH 6.0–10.0, and in the presence of 0–12.5% (w/v) NaCl; the optimum growth conditions were 25°C and pH 7.0, and the optimum Na+ concentration was 2.5%. In addition, strain M44 contained 18 : 1 ω7c, 11 methyl 18 : 1 ω7c and 16 : 0 fatty acids as major fatty acids, and the genomic DNA G+C content was 58.04 mol%. |

Cyclo(Phe-Val) Dilution Calculator

Cyclo(Phe-Val) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0599 mL | 20.2996 mL | 40.5992 mL | 81.1985 mL | 101.4981 mL |
| 5 mM | 0.812 mL | 4.0599 mL | 8.1198 mL | 16.2397 mL | 20.2996 mL |
| 10 mM | 0.406 mL | 2.03 mL | 4.0599 mL | 8.1198 mL | 10.1498 mL |
| 50 mM | 0.0812 mL | 0.406 mL | 0.812 mL | 1.624 mL | 2.03 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.406 mL | 0.812 mL | 1.015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2-4-Hydroxy-3-methoxyphenyl-7-methoxy-5-benzofuranpropanol
Catalog No.:BCN1564
CAS No.:144735-57-9
- 4F 4PP oxalate
Catalog No.:BCC6678
CAS No.:144734-36-1
- Telmisartan tert-butyl ester
Catalog No.:BCC9161
CAS No.:144702-26-1
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Fmoc-D-Cha-OH
Catalog No.:BCC3161
CAS No.:144701-25-7
- Hispidulin
Catalog No.:BCN6250
CAS No.:1447-88-7
- Olmesartan medoxomil
Catalog No.:BCC2143
CAS No.:144689-63-4
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- (+)-Lyoniresinol
Catalog No.:BCN6248
CAS No.:14464-90-5
- 22-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1565
CAS No.:144629-84-5
- GR 113808
Catalog No.:BCC7019
CAS No.:144625-51-4
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- Cyclo(Ala-Phe)
Catalog No.:BCN2411
CAS No.:14474-78-3
- Clopidogrel Related Compound B
Catalog No.:BCN2688
CAS No.:144750-52-7
- Brachynoside heptaacetate
Catalog No.:BCN6249
CAS No.:144765-80-0
- L-703,664 succinate
Catalog No.:BCC7437
CAS No.:144776-01-2
- 5-(4-(2-(5-Ethylpyridin-2-yl)ethoxy)benzylidene)thiazolidine-2,4-dione
Catalog No.:BCC8720
CAS No.:144809-28-9
- Deflazacort
Catalog No.:BCC8928
CAS No.:14484-47-0
- Alpiniaterpene A
Catalog No.:BCN7085
CAS No.:1448667-05-7
- Garjasmin
Catalog No.:BCN6251
CAS No.:144868-43-9
- Resiquimod (R-848)
Catalog No.:BCC4073
CAS No.:144875-48-9
- Junipediol B
Catalog No.:BCN6252
CAS No.:144881-19-6
- 4',9,9'-Trihydroxy-3'-methoxy-3,7'-epoxy-4,8'-oxyneolignan
Catalog No.:BCN1563
CAS No.:144881-21-0
- MRS 2693 trisodium salt
Catalog No.:BCC7386
CAS No.:1448858-83-0


