Olmesartan medoxomilAT1 receptor antagonist CAS# 144689-63-4 |
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Istaroxime hydrochloride
Catalog No.:BCC1661
CAS No.:374559-48-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144689-63-4 | SDF | Download SDF |
| PubChem ID | 130881 | Appearance | Powder |
| Formula | C29H30N6O6 | M.Wt | 558.6 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CS 866 | ||
| Solubility | DMSO : 50 mg/mL (89.51 mM; Need ultrasonic) | ||
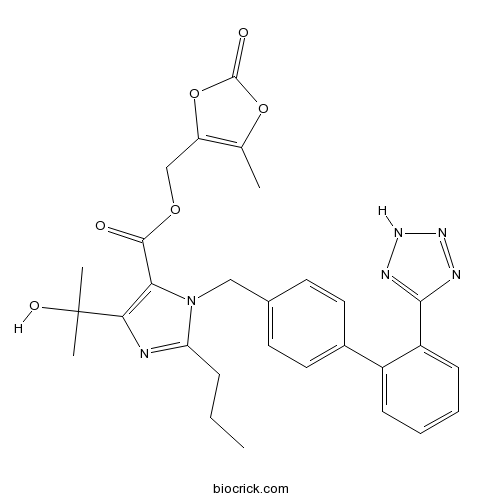
| Chemical Name | (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 5-(2-hydroxypropan-2-yl)-2-propyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]imidazole-4-carboxylate | ||
| SMILES | CCCC1=NC(=C(N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)C(=O)OCC5=C(OC(=O)O5)C)C(C)(C)O | ||
| Standard InChIKey | UQGKUQLKSCSZGY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H30N6O6/c1-5-8-23-30-25(29(3,4)38)24(27(36)39-16-22-17(2)40-28(37)41-22)35(23)15-18-11-13-19(14-12-18)20-9-6-7-10-21(20)26-31-33-34-32-26/h6-7,9-14,38H,5,8,15-16H2,1-4H3,(H,31,32,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Prodrug metabolised in vivo to Olmesartan, a selective non-peptide angiotensin II type I receptor (AT1) antagonist. Selectively inhibits angiotensin II binding to AT1 receptors in bovine adrenal cortical membranes (IC50 = 7.7 nM), with no effect on AT2 receptors. |

Olmesartan medoxomil Dilution Calculator

Olmesartan medoxomil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7902 mL | 8.9509 mL | 17.9019 mL | 35.8038 mL | 44.7547 mL |
| 5 mM | 0.358 mL | 1.7902 mL | 3.5804 mL | 7.1608 mL | 8.9509 mL |
| 10 mM | 0.179 mL | 0.8951 mL | 1.7902 mL | 3.5804 mL | 4.4755 mL |
| 50 mM | 0.0358 mL | 0.179 mL | 0.358 mL | 0.7161 mL | 0.8951 mL |
| 100 mM | 0.0179 mL | 0.0895 mL | 0.179 mL | 0.358 mL | 0.4475 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Olmesartan medoxomil, the medoxomil ester of olmesartan, is novel and oral antagonist of angiotensin II TYPE-1 receptor (AT1 receptor) with an inhibition constant IC50 of 33 nmol/L. Through oral administration, olmesartan medoxomil is rapidly absorbed by the gastrointestinal tract converting to its active metabolite olmesartan, whose inhibition constant IC50 towards AT1 receptor is 8 nmol/L. The esterification of olmesartan with medoxomil moiety is just to improve its water solubility (from 4.5% to 28.6%). Olmesartan medoxomil is used for the treatment of hypertension, in which olmesartan dose-dependently reduces the blood pressure through arterial vasodilation and reducing sodium retention.
Reference
HR Brunner. The new oral angiotensin II antagonist olmesartan medoxomil: a concise overview. Journal of Human Hypertension (2002) 16 (Suppl 2), S13-S16
Shailesh T. Prajapati, Hitesh H. Bulchandani, Dashrath M. Patel, Suresh K. Dumaniya and Chhaganbhai N. Patel. Formulation and evaluation of liquisolid compacts for olmesartan medoxomil. Journal of Drug Delivery 2013.
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- (+)-Lyoniresinol
Catalog No.:BCN6248
CAS No.:14464-90-5
- 22-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1565
CAS No.:144629-84-5
- GR 113808
Catalog No.:BCC7019
CAS No.:144625-51-4
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- Isomahanine
Catalog No.:BCN3177
CAS No.:144606-95-1
- Schisanlignone D
Catalog No.:BCN3630
CAS No.:144606-84-8
- Schisanlignone C
Catalog No.:BCN3629
CAS No.:144606-83-7
- IRL-1038
Catalog No.:BCC5730
CAS No.:144602-02-8
- Paliperidone
Catalog No.:BCC3834
CAS No.:144598-75-4
- Kanshone H
Catalog No.:BCN7627
CAS No.:1445952-33-9
- GSK 2837808A
Catalog No.:BCC5607
CAS No.:1445879-21-9
- Hispidulin
Catalog No.:BCN6250
CAS No.:1447-88-7
- Fmoc-D-Cha-OH
Catalog No.:BCC3161
CAS No.:144701-25-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Telmisartan tert-butyl ester
Catalog No.:BCC9161
CAS No.:144702-26-1
- 4F 4PP oxalate
Catalog No.:BCC6678
CAS No.:144734-36-1
- 2-4-Hydroxy-3-methoxyphenyl-7-methoxy-5-benzofuranpropanol
Catalog No.:BCN1564
CAS No.:144735-57-9
- Cyclo(Phe-Val)
Catalog No.:BCN2419
CAS No.:14474-71-6
- Cyclo(Ala-Phe)
Catalog No.:BCN2411
CAS No.:14474-78-3
- Clopidogrel Related Compound B
Catalog No.:BCN2688
CAS No.:144750-52-7
- Brachynoside heptaacetate
Catalog No.:BCN6249
CAS No.:144765-80-0
- L-703,664 succinate
Catalog No.:BCC7437
CAS No.:144776-01-2
- 5-(4-(2-(5-Ethylpyridin-2-yl)ethoxy)benzylidene)thiazolidine-2,4-dione
Catalog No.:BCC8720
CAS No.:144809-28-9
Development of olmesartan medoxomil optimized nanosuspension using the Box-Behnken design to improve oral bioavailability.[Pubmed:28271908]
Drug Dev Ind Pharm. 2017 Jul;43(7):1186-1196.
The aim of the present investigation was to enhance the oral bioavailability of Olmesartan medoxomil by improving its solubility and dissolution rate by preparing nanosuspension (OM-NS), using the Box-Behnken design. In this, four factors were evaluated at three levels. Independent variables include: concentration of drug (X1), concentration of surfactant (X2), concentration of polymer (X3) and number of homogenization cycles (X4). Based on preliminary studies, the size (Y1), zeta potential (ZP) (Y2) and % drug release at 5 min (Y3) were chosen as dependent responses. OM-NS was prepared by high pressure homogenization method. The size, PDI, ZP, assay, in vitro release and morphology of OM-NS were characterized. Further, the pharmacokinetic (PK) behavior of OM-NS was evaluated in male wistar rats. Statistically optimized OM-NS formulation exhibited mean particle size of 492 nm, ZP of -27.9 mV and 99.29% release in 5 min. OM-NS showed more than four times increase in its solubility than pure OM. DSC and XRD analyses indicated that the drug incorporated into OM-NS was in amorphous form. The morphology of OM-NS was found to be nearly spherical with high dispersity by scanning electron microscopic studies. The PK results showed that OM lyophilized nanosuspension (NS) exhibited improved PK properties compared to coarse powder suspension and marketed tablet powder suspension (TS). Oral bioavailability of lyophilized NS was increased by 2.45 and 2.25 folds when compared to marketed TS and coarse powder suspension, respectively. Results of this study lead to conclusion that NS approach was effective in preparing OM formulations with enhanced dissolution and improved oral bioavailability.
Development of olmesartan medoxomil lipid-based nanoparticles and nanosuspension: preparation, characterization and comparative pharmacokinetic evaluation.[Pubmed:28290712]
Artif Cells Nanomed Biotechnol. 2018 Feb;46(1):126-137.
The aim was to enhance the oral bioavailability of Olmesartan medoxomil (OM) by preparing solid lipid nanoparticles (SLNs) and comparing with nanosuspension (OM-NS). OM-SLNs and OM-NS were prepared by known methods. Prepared SLNs were evaluated for physical characters and in vivo pharmacokinetic (PK) performance in rats. OM-NS showed more than four-fold increase in the solubility. During DSC and XRD studies, drug incorporated in SLNs was found to be in amorphous form. The relative bioavailability of OM-SLN and OM-NS was 7.21- and 3.52-fold when compared with that of coarse suspension. Further, OM-SLNs also increased the oral bioavailability by two-fold over that of OM-NS.
Formulation, optimization, and in vitro-in vivo evaluation of olmesartan medoxomil nanocrystals.[Pubmed:28116656]
Drug Deliv Transl Res. 2017 Apr;7(2):292-303.
The aim of the present study is to increase the saturation solubility and oral bioavailability of Olmesartan medoxomil (OLM) using nano-sized crystals produced using a combination of antisolvent precipitation and high-shear homogenization. A response surface design comprising 46 runs was used to optimize the OLM nanocrystal formulation. The optimized formulation was produced using a combination of D-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS) (0.7% w/v), Pluronic F-68(R) (0.5% w/v), and drug concentration (0.2% w/v) and subjected to 10 and 15 homogenization cycles at 1000 and 1700 bar, respectively. The particle size, polydispersity index (PDI), and zeta potential of optimized formulation were found to be 140 +/- 10.34 nm, 0.07 +/- 0.016, and -21.43 +/- 2.33 mV, respectively. The optimized formulation exhibited irregular morphology as evaluated by scanning electron microscopy and was crystalline as determined by thermal analysis and powder X-ray diffraction studies. OLM nanocrystals showed a marked increase in the saturation solubility as well as rapid dissolution rate in comparison with the pure drug. No significant change in the particle size, PDI, and zeta potential was observed when optimized formulation was stored at room and refrigeration conditions for 3 months. Lastly, in vivo pharmacokinetic studies in Sprague-Dawley rats substantiate the ability of OLM nanocrystal formulation to significantly improve ( approximately 4.6-fold) the oral bioavailability of OLM in comparison with the free drug. This study has established a potential and commercial viable OLM formulation with enhanced saturation solubility and in vivo oral bioavailability.
Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability.[Pubmed:28284054]
Colloids Surf B Biointerfaces. 2017 Jun 1;154:10-20.
The current study explores the potential of nanostructured lipid carriers (NLCs) for oral bioavailability enhancement of Olmesartan medoxomil (OLM) by systemic design approach. OLM-NLC was successfully prepared with optimized process parameters (i.e. amount of liquid lipid, total amount of lipid, drug content and surfactant concentration) using the Box-Behnken design of experiments for different response parameters (i.e. particle size, Polydispersity index and entrapment efficiency). Further, optimized formulation was validated which depicted nano size, homogenous distribution with optimum entrapment efficiency. OLM-NLC was characterized by different techniques viz. differential scanning calorimetry (DSC), powder X-Ray diffraction (PXRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM), which showed reduced crystallinity of the drug with smooth spherical appearance of nanoparticles. Formulation was found to be stable in simulated gastric fluids as no significant changes were found in size, PDI and entrapment efficiency. In vitro release showed extended release of OLM from OLM-NLC. In vitro cellular uptake study revealed 5.2 folds higher uptake of nanoparticles as compare to the free drug, when incubated with Caco-2 cells. In vivo performance showed that AUCtotal and Cmax of OLM-NLC were found significantly (P<0.01) higher as compare to the free drug. Overall, the present study successfully reports the improvement of oral bioavailability of Olmesartan medoxomil.
Effects of angiotensin AT1 receptor antagonist on volume overload-induced cardiac gene expression in rats.[Pubmed:9220278]
Hypertens Res. 1997 Jun;20(2):133-42.
The present study was undertaken to examine the effects of volume overload on cardiac gene expression and the possible role of angiotensin AT1 receptor in such expression. Cardiac volume overload was prepared by abdominal aortocaval shunt in rats. Rats with aortocaval shunt were treated with 1) vehicle, 2) an angiotensin AT1 receptor antagonist, CS-866 (10 mg/kg/d), or 3) an angiotensin-converting enzyme inhibitor, temocapril (10 mg/kg/d), for 7 days. Cardiac tissue mRNA was measured by Northern blot analysis with specific probes. Aortocaval shunt not only caused cardiac hypertrophy but also upregulated the gene expression of atrial natriuretic polypeptide, collagen III, and downregulated Ca(2+)-ATPase expression in the left ventricle. These changes were prevented by treatment with CS-866, while temocapril failed to normalize left ventricular Ca(2+)-ATPase expression. Unlike the left ventricle, the significant downregulation of alpha-myosin heavy chain and transforming growth factor-beta 3 by aortocaval shunt was observed in the right ventricle, and CS-866 normalized this decreased expression of transforming growth factor-beta 3. The left and right atria showed increased expression of collagen type I as well as of collagen type III and atrial natriuretic polypeptide, and these increases were more effectively prevented by CS-866 than by temocapril. Thus, the effects of cardiac volume overload on cardiac performance-related gene expression differ between the ventricles and atria. Our results suggest that AT1 receptor partially contributed to volume overload-induced changes in cardiac gene expression and that AT1 receptor antagonists and angiotensin-converting enzyme inhibitors have different effects in this model of cardiac hypertrophy.
Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist.[Pubmed:8566137]
Eur J Pharmacol. 1995 Oct 16;285(2):181-8.
CS-866, (5-methyl-2-oxo-1,3-dioxolen-4-yl)methoxy-4-(1-hydroxy-1- methylethyl)-2-propyl-1-(4-[2-(tetrazol-5-yl)-phenyl]phenyl)met hylimidazol- 5-carboxylate, a prodrug type angiotensin receptor antagonist, is deesterified to the active acid, RNH-6270. RNH-6270 inhibited [125I]angiotensin II binding to bovine adrenal cortical membranes (angiotensin AT1 receptors) with an IC50 value of 7.7 nM, but not [125I]angiotensin II binding to bovine cerebellar membranes (angiotensin AT2 receptors), indicating the selectivity of the compound for angiotensin AT1 receptors. In guinea pig aortas, RNH-6270 reduced the maximal response of the concentration-contractile curve for angiotensin II (pD'2 = 9.9), but had no effect on the contractile response induced by phenylephrine or KCl. In conscious rats, intravenously injected RNH-6270 inhibited angiotensin II-induced pressor responses in a dose-dependent manner, and orally administered CS-866 produced a long-lasting inhibition of angiotensin II pressor responses. SK&F-525A, a P-450 inhibitor, suppressed the angiotensin II inhibitory effect of losartan, but not that of CS-866. These results demonstrate that RNH-6270 is a potent and AT1-selective angiotensin receptor antagonist and that, after oral administration, CS-866 has a long-lasting angiotensin II inhibitory action which is not affected by drug metabolizing enzymes in the liver.


