IsomahanineCAS# 144606-95-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144606-95-1 | SDF | Download SDF |
| PubChem ID | 375148 | Appearance | Powder |
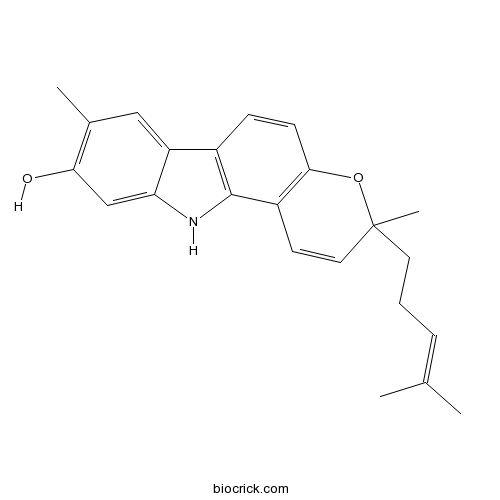
| Formula | C23H25NO2 | M.Wt | 347.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,8-dimethyl-3-(4-methylpent-3-enyl)-11H-pyrano[3,2-a]carbazol-9-ol | ||
| SMILES | CC1=C(C=C2C(=C1)C3=C(N2)C4=C(C=C3)OC(C=C4)(C)CCC=C(C)C)O | ||
| Standard InChIKey | WWXYBSVWYPHUPZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H25NO2/c1-14(2)6-5-10-23(4)11-9-17-21(26-23)8-7-16-18-12-15(3)20(25)13-19(18)24-22(16)17/h6-9,11-13,24-25H,5,10H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Isomahanine and mahanine show antibacterial activity towards Flavobacterium columnare and Streptococcus iniae which caused columnaris disease and streptococcosis respectively; isomahanine has the strongest activity against F. columnare (isolate ALM-00-173) and S. iniae (isolate LA94-426) based on 24-h 50% inhibition concentration (IC50) and minimum inhibition concentration (MIC). 2. Isomahanine is a potent inhibitor of sEH , with IC50 values of 22.565±651.7. |
| Targets | Antifection |

Isomahanine Dilution Calculator

Isomahanine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8777 mL | 14.3885 mL | 28.777 mL | 57.554 mL | 71.9424 mL |
| 5 mM | 0.5755 mL | 2.8777 mL | 5.7554 mL | 11.5108 mL | 14.3885 mL |
| 10 mM | 0.2878 mL | 1.4388 mL | 2.8777 mL | 5.7554 mL | 7.1942 mL |
| 50 mM | 0.0576 mL | 0.2878 mL | 0.5755 mL | 1.1511 mL | 1.4388 mL |
| 100 mM | 0.0288 mL | 0.1439 mL | 0.2878 mL | 0.5755 mL | 0.7194 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Schisanlignone D
Catalog No.:BCN3630
CAS No.:144606-84-8
- Schisanlignone C
Catalog No.:BCN3629
CAS No.:144606-83-7
- IRL-1038
Catalog No.:BCC5730
CAS No.:144602-02-8
- Paliperidone
Catalog No.:BCC3834
CAS No.:144598-75-4
- Kanshone H
Catalog No.:BCN7627
CAS No.:1445952-33-9
- GSK 2837808A
Catalog No.:BCC5607
CAS No.:1445879-21-9
- ME0328
Catalog No.:BCC3995
CAS No.:1445251-22-8
- Murrayanol
Catalog No.:BCN3178
CAS No.:144525-81-5
- Oxyresveratrol 3'-O-beta-D-glucopyranoside
Catalog No.:BCN1566
CAS No.:144525-40-6
- Isorhynchophyllic acid
Catalog No.:BCN4980
CAS No.:144525-05-3
- Pixantrone
Catalog No.:BCC5222
CAS No.:144510-96-3
- Cannabisin D
Catalog No.:BCN2547
CAS No.:144506-19-4
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- GR 113808
Catalog No.:BCC7019
CAS No.:144625-51-4
- 22-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1565
CAS No.:144629-84-5
- (+)-Lyoniresinol
Catalog No.:BCN6248
CAS No.:14464-90-5
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- Olmesartan medoxomil
Catalog No.:BCC2143
CAS No.:144689-63-4
- Hispidulin
Catalog No.:BCN6250
CAS No.:1447-88-7
- Fmoc-D-Cha-OH
Catalog No.:BCC3161
CAS No.:144701-25-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Telmisartan tert-butyl ester
Catalog No.:BCC9161
CAS No.:144702-26-1
- 4F 4PP oxalate
Catalog No.:BCC6678
CAS No.:144734-36-1
- 2-4-Hydroxy-3-methoxyphenyl-7-methoxy-5-benzofuranpropanol
Catalog No.:BCN1564
CAS No.:144735-57-9
Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii leaves.[Pubmed:14558763]
J Agric Food Chem. 2003 Oct 22;51(22):6461-7.
A new dimeric carbazole alkaloid, 8,10'-[3,3',11,11'-tetrahydro-9,9'-dihydroxy-3,3',5,8'-tetramethyl-3,3'-bis(4-met hyl-3-pentenyl)]bipyrano[3,2-a]carbazole (12), was isolated from the CH(2)Cl(2) extract of Murraya koenigii together with six known carbazole alkaloids, koenimbine (6), O-methylmurrayamine A (7), O-methylmahanine (8), Isomahanine (9), bismahanine (10), and bispyrayafoline (11). Their structures were determined on the basis of (1)H and (13)C NMR spectroscopic and mass spectrometric (MS) data. The antioxidative properties of 12 carbazole alkaloids isolated from leaves of M. koenigii were evaluated on the basis of the oil stability index together with their radical scavenging ability against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. On the basis of the lag time to reach a steady state, the 12 carbazoles were classified into three groups. It is suggested that an aryl hydroxyl substituent on the carbazole rings plays a role in stabilizing the thermal oxidation and rate of reaction against DPPH radical.


