ME0328PARP inhibitor,potent and selective CAS# 1445251-22-8 |
- MK-4827
Catalog No.:BCC1761
CAS No.:1038915-60-4
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- PJ34
Catalog No.:BCC1865
CAS No.:344458-19-1
- ABT-888 (Veliparib)
Catalog No.:BCC1267
CAS No.:912444-00-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1445251-22-8 | SDF | Download SDF |
| PubChem ID | 71571526 | Appearance | Powder |
| Formula | C19H19N3O2 | M.Wt | 321.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 75 mg/mL (233.38 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
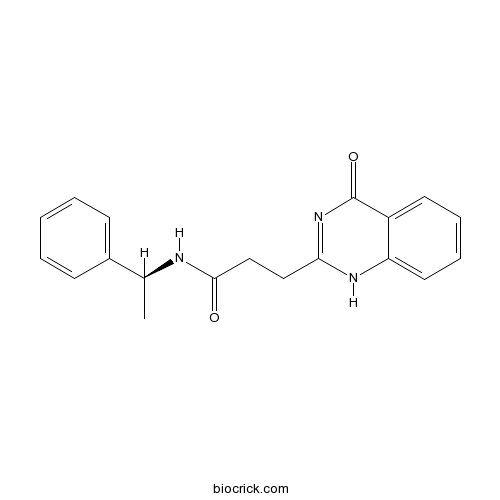
| Chemical Name | 3-(4-oxo-1H-quinazolin-2-yl)-N-[(1S)-1-phenylethyl]propanamide | ||
| SMILES | CC(C1=CC=CC=C1)NC(=O)CCC2=NC(=O)C3=CC=CC=C3N2 | ||
| Standard InChIKey | QIHBWVVVRYYYRO-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C19H19N3O2/c1-13(14-7-3-2-4-8-14)20-18(23)12-11-17-21-16-10-6-5-9-15(16)19(24)22-17/h2-10,13H,11-12H2,1H3,(H,20,23)(H,21,22,24)/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of PARP-3 (IC50 = 0.89 μM). Displays selectivity for PARP-3 over PARP-1, PARP-2 and other ARDT enzymes (IC50 values are 6.3, 10.8 and >30 μM respectively). Enhances CRISPR-Cas9-mediated HER2 mutation frequency, resulting in increased reduction in proliferation of HER2-positive breast cancer cells. Cell permeable. |

ME0328 Dilution Calculator

ME0328 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1117 mL | 15.5584 mL | 31.1168 mL | 62.2336 mL | 77.792 mL |
| 5 mM | 0.6223 mL | 3.1117 mL | 6.2234 mL | 12.4467 mL | 15.5584 mL |
| 10 mM | 0.3112 mL | 1.5558 mL | 3.1117 mL | 6.2234 mL | 7.7792 mL |
| 50 mM | 0.0622 mL | 0.3112 mL | 0.6223 mL | 1.2447 mL | 1.5558 mL |
| 100 mM | 0.0311 mL | 0.1556 mL | 0.3112 mL | 0.6223 mL | 0.7779 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ME0328 is a potent and selective inhibitor of PARP with IC50 value of 0.89 and 6.3 μM for PARP3 and PARP1, respectively [1].
Poly (ADP-ribose) polymerase (PARP) is a family of proteins mainly involved in programmed cell death and DNA repair. The overactivation of PARP may reduce the stores of cellular NAD+ and induce ATP depletion and cell death.
ME0328 is a potent and selective PARP inhibitor. ME0328 selectively inhibited ADP-ribosyltransferase-3/poly(ADP-ribose) polymerase-3 (ARTD3) with IC50 value of 0.89 μM and inhibited ARTD1 and ARTD2 with IC50 values of 6.3 and 10.8 μM, respectively. In human alveolar basal epithelial (A549) cells, treatment with ME0355 (10 μM) followed by γ-irradiation significantly delayed γH2AX-foci resolution in a concentration-dependent way, which suggested that ME0328 inhibited ARTD3 in cells. In nonirradiated human A549 and mouse MRC5 cells, ME0328 (10 μM) did not exhibit toxicity [1].
Reference:
[1]. Lindgren AE, Karlberg T, Thorsell AG, et al. PARP inhibitor with selectivity toward ADP-ribosyltransferase ARTD3/PARP3. ACS Chem Biol, 2013, 8(8): 1698-1703.
- Murrayanol
Catalog No.:BCN3178
CAS No.:144525-81-5
- Oxyresveratrol 3'-O-beta-D-glucopyranoside
Catalog No.:BCN1566
CAS No.:144525-40-6
- Isorhynchophyllic acid
Catalog No.:BCN4980
CAS No.:144525-05-3
- Pixantrone
Catalog No.:BCC5222
CAS No.:144510-96-3
- Cannabisin D
Catalog No.:BCN2547
CAS No.:144506-19-4
- Rotundatin
Catalog No.:BCN7856
CAS No.:144506-16-1
- Licochalcone C
Catalog No.:BCN6334
CAS No.:144506-14-9
- Tirofiban
Catalog No.:BCC4868
CAS No.:144494-65-5
- Clopidogrel Related Compound A
Catalog No.:BCN2687
CAS No.:144457-28-3
- Beta-Aflatrem
Catalog No.:BCN6699
CAS No.:144446-23-1
- Goniodiol 8-acetate
Catalog No.:BCN4787
CAS No.:144429-71-0
- Aflavarin
Catalog No.:BCN7410
CAS No.:144429-67-4
- GSK 2837808A
Catalog No.:BCC5607
CAS No.:1445879-21-9
- Kanshone H
Catalog No.:BCN7627
CAS No.:1445952-33-9
- Paliperidone
Catalog No.:BCC3834
CAS No.:144598-75-4
- IRL-1038
Catalog No.:BCC5730
CAS No.:144602-02-8
- Schisanlignone C
Catalog No.:BCN3629
CAS No.:144606-83-7
- Schisanlignone D
Catalog No.:BCN3630
CAS No.:144606-84-8
- Isomahanine
Catalog No.:BCN3177
CAS No.:144606-95-1
- CPI-203
Catalog No.:BCC4099
CAS No.:1446144-04-2
- GR 113808
Catalog No.:BCC7019
CAS No.:144625-51-4
- 22-Hydroxy-3-oxoolean-12-en-29-oic acid
Catalog No.:BCN1565
CAS No.:144629-84-5
- (+)-Lyoniresinol
Catalog No.:BCN6248
CAS No.:14464-90-5
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
PARP inhibitor with selectivity toward ADP-ribosyltransferase ARTD3/PARP3.[Pubmed:23742272]
ACS Chem Biol. 2013 Aug 16;8(8):1698-703.
Inhibiting ADP-ribosyl transferases with PARP-inhibitors is considered a promising strategy for the treatment of many cancers and ischemia, but most of the cellular targets are poorly characterized. Here, we describe an inhibitor of ADP-ribosyltransferase-3/poly(ADP-ribose) polymerase-3 (ARTD3), a regulator of DNA repair and mitotic progression. In vitro profiling against 12 members of the enzyme family suggests selectivity for ARTD3, and crystal structures illustrate the molecular basis for inhibitor selectivity. The compound is active in cells, where it elicits ARTD3-specific effects at submicromolar concentration. Our results show that by targeting the nicotinamide binding site, selective inhibition can be achieved among the closest relatives of the validated clinical target, ADP-ribosyltransferase-1/poly(ADP-ribose) polymerase-1.
CRISPR-mediated targeting of HER2 inhibits cell proliferation through a dominant negative mutation.[Pubmed:27815036]
Cancer Lett. 2017 Jan 28;385:137-143.
With the discovery of the CRISPR/Cas9 technology, genome editing could be performed in a rapid, precise and effective manner. Its potential applications in functional interrogation of cancer-causing genes and cancer therapy have been extensively explored. In this study, we demonstrated the use of the CRISPR/Cas9 system to directly target the oncogene HER2. Directing Cas9 to exons of the HER2 gene inhibited cell growth in breast cancer cell lines that harbor amplification of the HER2 locus. The inhibitory effect was potentiated with the addition of PARP inhibitors. Unexpectedly, CRISPR-induced mutations did not significantly affect the level of HER2 protein expression. Instead, CRISPR targeting appeared to exert its effect through a dominant negative mutation. This HER2 mutant interfered with the MAPK/ERK axis of HER2 downstream signaling. Our work provides a novel mechanism underlying the anti-cancer effects of HER2-targeting by CRISPR/Cas9, which is distinct from the clinical drug Herceptin. In addition, it opens up the possibility that incomplete CRISPR targeting of certain oncogenes could still have therapeutic value by generation of dominant negative mutants.


