Delphinidin-3-O-arabinosideCAS# 28500-01-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28500-01-8 | SDF | Download SDF |
| PubChem ID | 137347179.0 | Appearance | Powder |
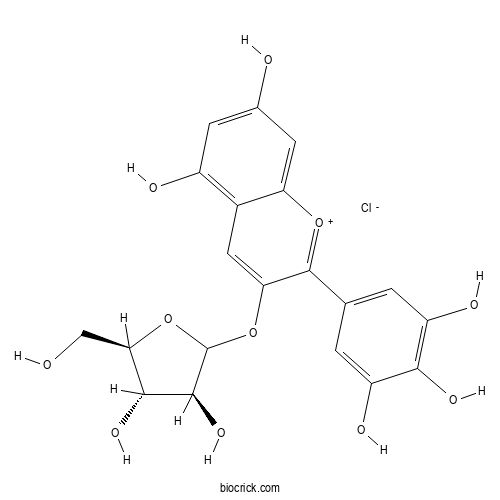
| Formula | C20H19ClO11 | M.Wt | 470.81 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-[3-[(3S,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-5,7-dihydroxychromenylium-2-yl]benzene-1,2,3-triol;chloride | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C2=[O+]C3=CC(=CC(=C3C=C2OC4C(C(C(O4)CO)O)O)O)O.[Cl-] | ||
| Standard InChIKey | GAULMASLJMFDEL-STHKZPLESA-N | ||
| Standard InChI | InChI=1S/C20H18O11.ClH/c21-6-15-17(27)18(28)20(31-15)30-14-5-9-10(23)3-8(22)4-13(9)29-19(14)7-1-11(24)16(26)12(25)2-7;/h1-5,15,17-18,20-21,27-28H,6H2,(H4-,22,23,24,25,26);1H/t15-,17-,18+,20?;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Delphinidin-3-O-arabinoside Dilution Calculator

Delphinidin-3-O-arabinoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.124 mL | 10.62 mL | 21.24 mL | 42.48 mL | 53.1 mL |
| 5 mM | 0.4248 mL | 2.124 mL | 4.248 mL | 8.496 mL | 10.62 mL |
| 10 mM | 0.2124 mL | 1.062 mL | 2.124 mL | 4.248 mL | 5.31 mL |
| 50 mM | 0.0425 mL | 0.2124 mL | 0.4248 mL | 0.8496 mL | 1.062 mL |
| 100 mM | 0.0212 mL | 0.1062 mL | 0.2124 mL | 0.4248 mL | 0.531 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyanidin3-O-2G-glucosylrutinoside
Catalog No.:BCX0878
CAS No.:755695-57-9
- Cyanidin-3-Rutinosidechloride Cyanidin-3-Rutinoside
Catalog No.:BCX0877
CAS No.:28338-59-2
- Ilexgenin B
Catalog No.:BCX0876
CAS No.:109008-39-1
- Quercitin-3′-O- glucofuranoside
Catalog No.:BCX0875
CAS No.:21637-25-2
- Demethoxydeacetoxypseudolaric acid B
Catalog No.:BCX0874
CAS No.:500736-17-4
- Breviscapine
Catalog No.:BCX0873
CAS No.:116122-36-2
- Celosin K
Catalog No.:BCX0872
CAS No.:1950579-53-9
- N-acetyldopamine dimmers A
Catalog No.:BCX0871
CAS No.:1519015-73-6
- Lugrandoside
Catalog No.:BCX0870
CAS No.:117457-37-1
- Acetyl Dopamine DimerIII
Catalog No.:BCX0869
CAS No.:916888-49-8
- α-Curcumene
Catalog No.:BCX0868
CAS No.:644-30-4
- Deacetylkadsurin
Catalog No.:BCX0867
CAS No.:51670-42-9
- Delphinidin-3-Rutinoside Delphinidin-3-Rutinosidechloride
Catalog No.:BCX0880
CAS No.:58285-26-0
- GalantamineHydrobromide
Catalog No.:BCX0881
CAS No.:69353-21-5
- (+)-Medicarpin
Catalog No.:BCX0882
CAS No.:33983-40-3
- Benzoylalbiflorin
Catalog No.:BCX0883
CAS No.:184103-78-4
- Allosecurinin
Catalog No.:BCX0884
CAS No.:884-68-4
- Lucenin-1 Luteolin-6-C-β-D-xylopyranoside-8-C-β-D-glucopyranosyl
Catalog No.:BCX0885
CAS No.:35927-39-0
- Lucenin-3 Luteolin-6-C-β-D-glucopyranosyl-8-C-β-D-xylopyranoside
Catalog No.:BCX0886
CAS No.:12656-83-6
- IsovitexinApigenin6-C-glucoside
Catalog No.:BCX0887
CAS No.:29702-25-8
- Delphinidin3-O-(6''-coumaroylglucoside)
Catalog No.:BCX0888
CAS No.:136031-08-8
- peonidin-3-O-(6''-coumaroyl)glucoside-5-O-glucoside
Catalog No.:BCX0889
CAS No.:205505-30-2
- Peonidin3-O-(6-O-p-coumaroyl)glucoside
Catalog No.:BCX0890
CAS No.:305833-55-0
- petunidin-3-O-(6"-O-p-coumaroyl)glucoside-5-O-diglucoside
Catalog No.:BCX0891
CAS No.:1063818-55-2
Metabolomic analysis to unravel the composition and dynamic variations of anthocyanins in bayberry-soaked wine during the maceration process.[Pubmed:38379795]
Food Chem X. 2024 Feb 6;21:101175.
In this work, we employed a global untargeted metabolomics technique to explore the intricate composition of anthocyanin constituents in bayberry wine and elucidate their alteration during the maceration process. Our analysis uncovered 20 distinct forms of anthocyanins in bayberry wine, including cyanidin-type, delphinidin-type, peonidin-type, malvidin-type, and other-type. 'Dongkui' (DK) bayberry wine was characterized by a predominance of glycoside forms of cyanidin-type and delphinidin-type anthocyanins, while 'Shuijing' (SJ) bayberry wine mainly contained other-type anthocyanins. Additionally, differential anthocyanins analyses conducted across various maceration periods demonstrated the different fate of the components in the wine, with a conspicuous decline in most glycosidic form anthocyanins. Moreover, correlation analysis revealed that the red hue of bayberry wine was primarily associated with cyanidin-3-O-glucoside, cyanidin-3-O-rhamnoside, Delphinidin-3-O-arabinoside, and delphinidin-3-O-galactoside. This research contributes to our understanding of the anthocyanin composition and the dynamic variations in bayberry wine, opening avenues for further exploration and optimization of production techniques in the future.
Metabolomic and transcriptomic analyses of the flavonoid biosynthetic pathway in blueberry (Vaccinium spp.).[Pubmed:37152168]
Front Plant Sci. 2023 Apr 20;14:1082245.
As a highly economic small fruit crop, blueberry is enjoyed by most people in terms of color, taste, and rich nutrition. To better understand its coloring mechanism on the process of ripening, an integrative analysis of the metabolome and transcriptome profiles was performed in three blueberry varieties at three developmental stages. In this study, 41 flavonoid metabolites closely related to the coloring in blueberry samples were analyzed. It turned out that the most differential metabolites in the ripening processes were Delphinidin-3-O-arabinoside (dpara), peonidin-3-O-glucoside (pnglu), and delphinidin-3-O-galactoside (dpgal), while the most differential metabolites among different varieties were flavonols. Furthermore, to obtain more accurate and comprehensive transcripts of blueberry during the developmental stages, PacBio and Illumina sequencing technology were combined to obtain the transcriptome of the blueberry variety Misty, for the very first time. Finally, by applying the gene coexpression network analysis, the darkviolet and bisque4 modules related to flavonoid synthesis were determined, and the key genes related to two flavonoid 3', 5'-hydroxylase (F3'5'H) genes in the darkviolet module and one bHLH transcription factor in the bisque4 module were predicted. It is believed that our findings could provide valuable information for the future study on the molecular mechanism of flavonoid metabolites and flavonoid synthesis pathways in blueberries.
Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (vaccinium ashei: Reade).[Pubmed:35488209]
BMC Plant Biol. 2022 Apr 29;22(1):223.
BACKGROUND: Blueberry is one of the most important fruit crops worldwide. Anthocyanin is an important secondary metabolites that affects the appearance and nutritive quality of blueberries. However, few studies have focused on the molecular mechanism underlying anthocyanin accumulation induced by light intensity in blueberries. RESULTS: The metabolic analysis revealed that there were 134 significantly changed metabolites in the natural light compared to the control, and flavone, flavonol, and anthocyanins were the most significantly increased. Transcriptome analysis found 6 candidate genes for the anthocyanin synthesis pathway. Quantitative reverse transcription PCR (qRT-PCR) results confirmed changes in the expression levels of genes encoding metabolites involved in the flavonoid synthesis pathways. The flavonoid metabolic flux in the light intensity-treatment increased the accumulation of Delphinidin-3-O-arabinoside compared to under the shading-treatment. Furthermore, we performed qRT-PCR analysis of anthocyanin biosynthesis genes and predicted that the gene of VcF3'5'H4 may be a candidate gene for anthocyanin accumulation and is highly expressed in light intensity-treated fruit. Through the co-expression analysis of transcription factors and anthocyanin synthesis pathway genes, we found that the VcbHLH004 gene may regulate VcF3'5'H4, and then we transformed VcbHLH004 heterologously into tomato to verify its function. CONCLUSION: These results provide novel insights into light intensity regulation of blueberry anthocyanin accumulation and represent a valuable data set to guide future functional studies and blueberry breeding.
Identification of Anthocyanins and Their Fouling Mechanisms during Non-Thermal Nanofiltration of Blueberry Aqueous Extracts.[Pubmed:33809170]
Membranes (Basel). 2021 Mar 12;11(3):200.
Organic fouling in the nanofiltration (NF) process, which is a non-thermal technology to recover active components, is a critical problem limiting its applications. This study seeks to identify the anthocyanins on the NF membrane and explore their fouling mechanisms during concentration of blueberry extracts. Seven kinds of monomeric anthocyanins in foulants-delphinidin-3-O-galactoside, delphinidin-3-O-glucoside, Delphinidin-3-O-arabinoside, cyanidin-3-O-galactoside, petunidin-3-O-galactoside, peonidin-3-O-glucoside, and malvidin-3-O-glucoside-were identified. Moreover, chalcone, myricetin derivative, and an unknown substance with [M(+)H](+) at m/z 261.1309, which is the fragment ion corresponding to the break of glycoside bond of anthocyanins, were obtained. Interactions between anthocyanins and membrane made from polyamide were principally governed by the CH-pi and pi-pi stacking of aromatic rings, the establishment of hydrogen bonds, and electrostatic interaction. This study will be helpful to further control fouling and choice of cleaning agents in concentration of anthocyanins-rich extracts.
Berry Phenolic Compounds Increase Expression of Hepatocyte Nuclear Factor-1alpha (HNF-1alpha) in Caco-2 and Normal Colon Cells Due to High Affinities with Transcription and Dimerization Domains of HNF-1alpha.[Pubmed:26413797]
PLoS One. 2015 Sep 28;10(9):e0138768.
Hepatocyte nuclear factor-1alpha (HNF-1alpha) is found in the kidneys, spleen, thymus, testis, skin, and throughout the digestive organs. It has been found to promote the transcription of various proteins involved in the management of type II diabetes, including dipeptidyl peptidase-IV (DPP-IV). Phenolic compounds from berries and citrus fruits are known to inhibit DPP-IV, but have not been tested for their interactions with wild-type HNF-1alpha. By studying the interactions of compounds from berries and citrus fruits have with HNF-1alpha, pre-transcriptional mechanisms that inhibit the expression of proteins such as DPP-IV may be elucidated. In this study, the interactions of berry phenolic compounds and citrus flavonoids with the dimerization and transcriptional domains of HNF-1alpha were characterized using the molecular docking program AutoDock Vina. The anthocyanin Delphinidin-3-O-arabinoside had the highest binding affinity for the dimerization domain as a homodimer (-7.2 kcal/mol) and transcription domain (-8.3 kcal/mol) of HNF-1alpha. Anthocyanins and anthocyanidins had relatively higher affinities than resveratrol and citrus flavonoids for both, the transcription domain and the dimerization domain as a homodimer. The flavonoid flavone had the highest affinity for a single unit of the dimerization domain (-6.5 kcal/mol). Nuclear expression of HNF-1alpha was measured in Caco-2 and human normal colon cells treated with blueberry and blackberry anthocyanin extracts. All extracts tested increased significantly (P < 0.05) the nuclear expression of HNF-1alpha in Caco-2 cells by 85.2 to 260% compared to a control. The extracts tested increased significantly (P < 0.02) the nuclear expression of HNF-1alpha in normal colon cells by 48.6 to 243%. It was confirmed that delphinidin-3-O-glucoside increased by 3-fold nuclear HNF-1alpha expression in Caco-2 cells (P < 0.05). Anthocyanins significantly increased nuclear HNF-1alpha expression, suggesting that these compounds might regulate the genes HNF-1alpha promotes.


