BenzoylalbiflorinCAS# 184103-78-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 184103-78-4 | SDF | Download SDF |
| PubChem ID | 163285795.0 | Appearance | Powder |
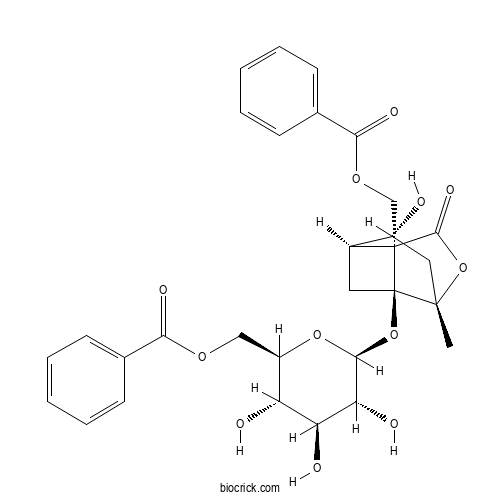
| Formula | C30H32O12 | M.Wt | 584.57 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2R,3S,4S,5R,6S)-6-[[(1S,3R,4R,6S,9S)-9-(benzoyloxymethyl)-4-hydroxy-6-methyl-8-oxo-7-oxatricyclo[4.3.0.03,9]nonan-1-yl]oxy]-3,4,5-trihydroxyoxan-2-yl]methyl benzoate | ||
| SMILES | CC12CC(C3CC1(C3(C(=O)O2)COC(=O)C4=CC=CC=C4)OC5C(C(C(C(O5)COC(=O)C6=CC=CC=C6)O)O)O)O | ||
| Standard InChIKey | ZHQGREQIJCCKHT-JXBNAFTOSA-N | ||
| Standard InChI | InChI=1S/C30H32O12/c1-28-13-19(31)18-12-30(28,29(18,27(37)42-28)15-39-25(36)17-10-6-3-7-11-17)41-26-23(34)22(33)21(32)20(40-26)14-38-24(35)16-8-4-2-5-9-16/h2-11,18-23,26,31-34H,12-15H2,1H3/t18-,19+,20+,21+,22-,23+,26-,28-,29-,30+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzoylalbiflorin Dilution Calculator

Benzoylalbiflorin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7107 mL | 8.5533 mL | 17.1066 mL | 34.2132 mL | 42.7665 mL |
| 5 mM | 0.3421 mL | 1.7107 mL | 3.4213 mL | 6.8426 mL | 8.5533 mL |
| 10 mM | 0.1711 mL | 0.8553 mL | 1.7107 mL | 3.4213 mL | 4.2766 mL |
| 50 mM | 0.0342 mL | 0.1711 mL | 0.3421 mL | 0.6843 mL | 0.8553 mL |
| 100 mM | 0.0171 mL | 0.0855 mL | 0.1711 mL | 0.3421 mL | 0.4277 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Medicarpin
Catalog No.:BCX0882
CAS No.:33983-40-3
- GalantamineHydrobromide
Catalog No.:BCX0881
CAS No.:69353-21-5
- Delphinidin-3-Rutinoside Delphinidin-3-Rutinosidechloride
Catalog No.:BCX0880
CAS No.:58285-26-0
- Delphinidin-3-O-arabinoside
Catalog No.:BCX0879
CAS No.:28500-01-8
- Cyanidin3-O-2G-glucosylrutinoside
Catalog No.:BCX0878
CAS No.:755695-57-9
- Cyanidin-3-Rutinosidechloride Cyanidin-3-Rutinoside
Catalog No.:BCX0877
CAS No.:28338-59-2
- Ilexgenin B
Catalog No.:BCX0876
CAS No.:109008-39-1
- Quercitin-3′-O- glucofuranoside
Catalog No.:BCX0875
CAS No.:21637-25-2
- Demethoxydeacetoxypseudolaric acid B
Catalog No.:BCX0874
CAS No.:500736-17-4
- Breviscapine
Catalog No.:BCX0873
CAS No.:116122-36-2
- Celosin K
Catalog No.:BCX0872
CAS No.:1950579-53-9
- N-acetyldopamine dimmers A
Catalog No.:BCX0871
CAS No.:1519015-73-6
- Allosecurinin
Catalog No.:BCX0884
CAS No.:884-68-4
- Lucenin-1 Luteolin-6-C-β-D-xylopyranoside-8-C-β-D-glucopyranosyl
Catalog No.:BCX0885
CAS No.:35927-39-0
- Lucenin-3 Luteolin-6-C-β-D-glucopyranosyl-8-C-β-D-xylopyranoside
Catalog No.:BCX0886
CAS No.:12656-83-6
- IsovitexinApigenin6-C-glucoside
Catalog No.:BCX0887
CAS No.:29702-25-8
- Delphinidin3-O-(6''-coumaroylglucoside)
Catalog No.:BCX0888
CAS No.:136031-08-8
- peonidin-3-O-(6''-coumaroyl)glucoside-5-O-glucoside
Catalog No.:BCX0889
CAS No.:205505-30-2
- Peonidin3-O-(6-O-p-coumaroyl)glucoside
Catalog No.:BCX0890
CAS No.:305833-55-0
- petunidin-3-O-(6"-O-p-coumaroyl)glucoside-5-O-diglucoside
Catalog No.:BCX0891
CAS No.:1063818-55-2
- Malvidin3-O-(6''-coumaroyl)glucoside
Catalog No.:BCX0892
CAS No.:158189-28-7
- Malvidin-3-O-(6''-O-coumaroyl)glucoside-5-O-glucoside
Catalog No.:BCX0893
CAS No.:144940-56-7
- Malvidin-3-(6-caffeoyl-glucoside)-5-glucoside
Catalog No.:BCX0894
CAS No.:1374753-08-8
- Eucomicacid
Catalog No.:BCX0895
CAS No.:60449-48-1
Chemical Profiling and Antioxidant Evaluation of Paeonia lactiflora Pall. "Zhongjiang" by HPLC-ESI-MS Combined with DPPH Assay.[Pubmed:33558884]
J Chromatogr Sci. 2021 Sep 29;59(9):795-805.
Paeonia lactiflora Pall. "Zhongjiang" is one of the four major medicinal P. lactiflora plants in China. In this research, a high-performance liquid chromatography (HPLC)-diode array detector (DAD)-electrospray ionization-mass spectrometry method was established to identify various components in the extracts of P. lactiflora "Zhongjiang" (root extract or RE, stem and leaf extract or SLE and flower extract or FE). A total of 40 compounds, including 19 monoterpenoid glycosides, five tannins, 10 phenolic acids and their esters, and six other compounds, were determined or temporarily inferred from RE (35 species), SLE (20 species) and FE (15 species). Antioxidant evaluation indicates among the monomer compounds, catechin, gallic acid and ethyl gallate showed strong antioxidant activity close to vitamin C, ascorbic acid (Vc). Paeoniflorin, albiflorin, benzoylpaeoniflorin and 6'-O-Benzoylalbiflorin had certain antioxidant activities, which were much lower than Vc. Furthermore, 19, 15 and 15 antioxidant-reactive components were screened from RE, SLE and FE by using the 1,1-diphenyl-2- picrylhydrazyl (DPPH)-HPLC test results. Results indicated that the ethanol extracts of P. lactiflora "Zhongjiang" had strong antioxidant activity, and the antioxidant active material basis was mainly composed of phenolic acids and gallic acid tannins. The main components of P. lactiflora "Zhongjiang", monoterpenoid glycosides, had weak antioxidant capacity. Paeonia lactiflora stems, leaves and flowers were good sources of antioxidants.
[Chemical constituents from water-soluble extract of dry roots of Paeonia lactiflora].[Pubmed:30111055]
Zhongguo Zhong Yao Za Zhi. 2018 Jul;43(14):2956-2963.
Nineteen compounds were isolated from the water-soluble extract of the dry roots of Paeonia lactiflora by using various chromatographic techniques. Their structures were identified by MS, NMR and other spectroscopic analysis as paeoniflorin(1), 4-O-ethylpaeoniflorin(2), 2'-O-benzoylpaeoniflorin(3), benzoylpaeoniflorin(4), 4"-hydroxy-benzoyloxypaeoniflorin(5), moudanpioside C(6), 6'-O-benzoyl-4"-hydroxy-3"-methoxy-paeoniflorin(7), paeoniflorin B(8), 6-O-Benzoylalbiflorin(9), secoisolariciresinol (10), (+)-lyoniresinol(11), dihyrodehydrodiconiferyl alcohol(12), (7S,8S)-threo-7,9,9'-trihydroxy-3,3'-dimethoxy-8-O-4'-neolignan(13), (+)-neo-olivil (14), [(3S)-5-methyl-2,3-dihydro-1-benzofuran-3-yl]methanol(15), 5-hydroxy-3S-hydroxymethyl-6-methyl-2,3-dihydrobenzofuran(16), (+)-(R)-2-hydroxy-1-(4-methoxyphenyl)-1-propan-1-one(17), (+)-(2R)-1-(4-hydroxy-3-methoxyphenyl)-2-propanol(18), (+)-(4S)-(2E)-4-hydroxy-2-nonenoic acid(19). Compounds 15 and 18 are new natural products, while compounds 10, 11, 13, 14, 17 and 19 are isolated from the genus Paeonia for the first time.
Rapid Determination of Isomeric Benzoylpaeoniflorin and Benzoylalbiflorin in Rat Plasma by LC-MS/MS Method.[Pubmed:28567056]
Int J Anal Chem. 2017;2017:1693464.
Benzoylpaeoniflorin (BP) is a potential therapeutic agent against oxidative stress related Alzheimer's disease. In this study, a more rapid, selective, and sensitive liquid chromatography-tandem mass spectrometric (LC-MS/MS) method was developed to determine BP in rat plasma distinguishing with a monoterpene isomer, Benzoylalbiflorin (BA). The method showed a linear response from 1 to 1000 ng/mL (r > 0.9950). The precision of the interday and intraday ranged from 2.03 to 12.48% and the accuracy values ranged from -8.00 to 10.33%. Each running of the method could be finished in 4 minutes. The LC-MS/MS method was validated for specificity, linearity, precision, accuracy, recovery, and stability and was found to be acceptable for bioanalytical application. Finally, this fully validated method was successfully applied to a pharmacokinetic study in rats following oral administration.
Anti-Inflammatory Effects, SAR, and Action Mechanism of Monoterpenoids from Radix Paeoniae Alba on LPS-Stimulated RAW 264.7 Cells.[Pubmed:28468284]
Molecules. 2017 Apr 29;22(5):715.
Nine monoterpenoids from Radix Paeoniae Alba, including paeoniflorin derivatives, paeoniflorin (PF), 4-O-methylpaeoniflorin (MPF), 4-O-methylbenzoylpaeoniflorin (MBPF); paeonidanin derivatives, paeonidanin (PD), paeonidanin A (PDA), albiflorin derivatives, albiflorin (AF), Benzoylalbiflorin (BAF), galloylalbiflorin (GAF), and deBenzoylalbiflorin (DAF), were obtained in our previous phytochemistry investigations. Their anti-inflammatory effects were determined in the present study. The expression and production of pro-inflammatory cytokines in lipopolysaccharides (LPS)-stimulated RAW 264.7 cells were measured using an Elisa assay and nitric oxide (NO) release was determined using the Griess method. The results demonstrated that the most of the monoterpenoids suppressed the LPS-induced production of NO, interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-alpha). The anti-inflammatory activities of these monoterpenoids were closely related to their structural characteristics. Paeoniflorins and paeonidanins presented stronger anti-inflammatory activities than those of albiflorin derivatives. Furthermore, the action mechanisms of MBPF, having a strong anti-inflammatory effect, were investigated using quantitative reverse transcription polymerase chain reaction (RT-PCR) and Western blot methods. The results indicated that MBPF could down-regulate the mRNA and protein expression level of inducible nitric oxide synthase (iNOS) in LPS-stimulated RAW 264.7 cells. The mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K)/AKT and nuclear factor kappaB (NF-kappaB) signaling pathways are involved in mediating the role of MBPF in suppressing the expression and production of pro-inflammatory cytokines in RAW 264.7 cells.
A new monoterpene glycoside from the roots of Paeonia lactiflora increases the differentiation of osteoblastic MC3T3-E1 cells.[Pubmed:18038896]
Arch Pharm Res. 2007 Oct;30(10):1179-85.
A new monoterpene glycoside, 6'-O-beta-D-glucopyranosylalbiflorin (1), and four known compounds; albiflorin (2), 6'-O-Benzoylalbiflorin (3), paeoniflorin (4) and benzoyl paeoniflorin (5), were isolated from the methanolic extract of the roots of Paeonia lactiflora Pall.. Their chemical structures were completely elucidated using a combination of 2D NMR techniques (COSY, HMQC and HMBC) and HRESI-MS analyses. To investigate the bioactivities of these compounds, their effects on the differentiation of osteoblastic MC3T3-E1 cells were tested. Compound 1 (0.01-10 microM) significantly increased the alkaline phosphatase activity and nodules mineralization of MC3T3-E1 cells compared to those of the control (P<0.05). These results suggest that newly isolated compound 1 has a direct stimulatory effect on bone formation in vitro and may contribute to the prevention for osteoporosis.
Analysis of sodium adduct paeoniflorin, albiflorin and their derivatives by (+)ESI-MSn, DFT calculations and computer-aided mass spectrometry analysis program.[Pubmed:17199256]
J Mass Spectrom. 2007 Mar;42(3):335-45.
In order to analyze paeoniflorin, albiflorin and their derivatives (PADs) in Paeonia Lactiflora rapidly and effectively, (+)ESI-MS(n) experiments were conducted, from which two diagnostic fragment patterns were acquired. Meanwhile, the dehydration ability of aglycones of PADs was obtained by calculating their activation energy using density functional theory, through which the unique dehydration phenomenon of Benzoylalbiflorin, compared with benzoylpaeoniflorin, was interpreted. In addition, a computer-aided mass spectrometry analysis program was developed to facilitate the analysis of the unknown compound by suggesting the possible structure of the analyte.
Monoterpenoid derivatives from Paeonia delavayi.[Pubmed:12067159]
J Asian Nat Prod Res. 2002 Jun;4(2):135-40.
Three new monoterpene glycosides, 4-O-ethylpaeoniflorin (1), 6'-O-benzoyl-4''-hydroxy-3"-methoxy-paeoniflorin (2), 6'-O-Benzoylalbiflorin (3), and a new monoterpenoid, 9-hydroxy-paeonilactone-A (4) were isolated from the root cortex of Paeonia delavayi. Their structures were elucidated on the basis of spectral methods.


