DihydropinosylvinCAS# 14531-52-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14531-52-3 | SDF | Download SDF |
| PubChem ID | 442700 | Appearance | Powder |
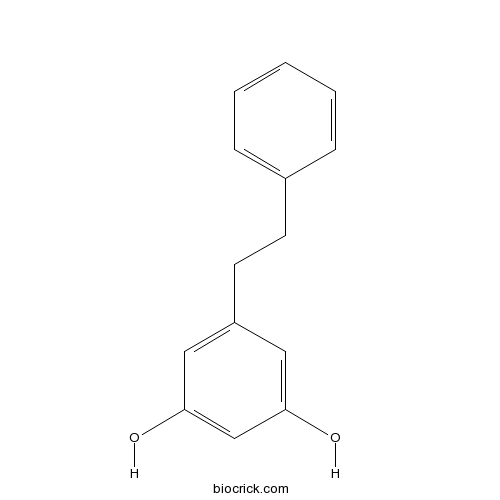
| Formula | C14H14O2 | M.Wt | 214.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-(2-phenylethyl)benzene-1,3-diol | ||
| SMILES | C1=CC=C(C=C1)CCC2=CC(=CC(=C2)O)O | ||
| Standard InChIKey | LDBYHULIXFIJAZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H14O2/c15-13-8-12(9-14(16)10-13)7-6-11-4-2-1-3-5-11/h1-5,8-10,15-16H,6-7H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dihydropinosylvinin is an phytoalexin, it shows antifungal activity against Cladosporium cladosporioides, Botryodiplodia theobromae, Aspergillus niger and Penicillium schlerotgenum, it also exhibits strong antibacterial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. 2. Dihydropinosylvin and batatasin IV can inhibit the germination of seeds of and root elongation in young seedlings of Sorghum bicolor. |
| Targets | Antifection |

Dihydropinosylvin Dilution Calculator

Dihydropinosylvin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6664 mL | 23.3318 mL | 46.6636 mL | 93.3271 mL | 116.6589 mL |
| 5 mM | 0.9333 mL | 4.6664 mL | 9.3327 mL | 18.6654 mL | 23.3318 mL |
| 10 mM | 0.4666 mL | 2.3332 mL | 4.6664 mL | 9.3327 mL | 11.6659 mL |
| 50 mM | 0.0933 mL | 0.4666 mL | 0.9333 mL | 1.8665 mL | 2.3332 mL |
| 100 mM | 0.0467 mL | 0.2333 mL | 0.4666 mL | 0.9333 mL | 1.1666 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bergenin pentaacetate
Catalog No.:BCN6257
CAS No.:14531-47-6
- TC-E 5002
Catalog No.:BCC5608
CAS No.:1453071-47-0
- A 77636 hydrochloride
Catalog No.:BCC7159
CAS No.:145307-34-2
- 20(R)-Protopanaxatriol
Catalog No.:BCN1079
CAS No.:1453-93-6
- Diarylcomosol III
Catalog No.:BCN7201
CAS No.:1452487-93-2
- Sahandol
Catalog No.:BCN6996
CAS No.:1452398-07-0
- Delgrandine
Catalog No.:BCN8122
CAS No.:145237-05-4
- Clobenpropit dihydrobromide
Catalog No.:BCC6781
CAS No.:145231-35-2
- CALP1
Catalog No.:BCC5873
CAS No.:145224-99-3
- Punctanecine
Catalog No.:BCN2018
CAS No.:145204-91-7
- Rizatriptan Benzoate
Catalog No.:BCC3852
CAS No.:145202-66-0
- Kaliotoxin
Catalog No.:BCC7710
CAS No.:145199-73-1
- Isochlorogenic acid B
Catalog No.:BCN5909
CAS No.:14534-61-3
- Sideroxylonal A
Catalog No.:BCN1645
CAS No.:145382-68-9
- GDC-0994
Catalog No.:BCC6371
CAS No.:1453848-26-4
- 1,4,7-Eudesmanetriol
Catalog No.:BCN1646
CAS No.:145400-02-8
- Homalomenol A
Catalog No.:BCN1647
CAS No.:145400-03-9
- L-692,585
Catalog No.:BCC7305
CAS No.:145455-35-2
- Heterophyllin B
Catalog No.:BCN2768
CAS No.:145459-19-4
- Isolintetralin
Catalog No.:BCN3052
CAS No.:145459-30-9
- PU-WS13
Catalog No.:BCC6425
CAS No.:1454619-14-7
- Furano(2'',3'',7,6)-4'-hydroxyflavanone
Catalog No.:BCN6405
CAS No.:1454619-70-5
- LY3009120
Catalog No.:BCC3985
CAS No.:1454682-72-4
- 4-Benzoylpyridine
Catalog No.:BCC8697
CAS No.:14548-46-0
[Chemical constitunents of seeds of Oroxylum indicum].[Pubmed:23672042]
Zhongguo Zhong Yao Za Zhi. 2013 Jan;38(2):204-7.
OBJECTIVE: To study the chemical constituents in the seeds of Oroxylum indicum. METHOD: Twenty compounds were isolated and purified by silica gel, and Sephadex LH-20 column chromatography, and their structures were determined by spectroscopic analysis including NMR and MS. RESULT: Twenty compounds were isolated and identified as oroxin A (1), oroxin B (2), chrysin (3), baicalein (4), quercetin (5), apigenin (6), kaempferol (7), quercetin-3-O-ara-binopyranoside (8), lupeol C9), lup-20 (29)-ene-2alpha,3beta-diol (10), pinosylvin (11), Dihydropinosylvin (12), cholest-5-ene-3, 7-diol (13), rengyol (14), isorengyol (15), zarzissine (16), (E) -pinosylvin-3-O-beta-D-glucopyranoside (17), adenosine (18), sitosterol (19) and daucosterol (20). CONCLUSION: Compounds 11-13 and 15-18 were obtained from the genus Oroxylum for the first time, and except compound 18, the remaining 6 compounds were obtained from the family Bignoniaceae for the first time.
Dihydrophenanthrenes and other antifungal stilbenoids from Stemona cf. pierrei.[Pubmed:14697275]
Phytochemistry. 2004 Jan;65(1):99-106.
Three new dihydrophenanthrenes, stemanthrenes A-C, along with the new dihydrostilbene stilbostemin G were isolated and identified from the underground parts of Stemona cf. pierrei together with the known pinosylvin, 4'-methylpinosylvin, Dihydropinosylvin, stilbostemins B, D, and E as well as the pyrrolo[1,2-a]azepine alkaloids protostemonine and stemonine. The structures of all new stilbenoids, elucidated by NMR analyses, showed a common substitution pattern for aromatic ring A and characteristic C-methylations for ring B. The trivial name racemosol, previously reported for S. collinsae, was renamed to stemanthrene D due to its priority for another compound. Bioautographic tests on TLC plates with Cladosporium herbarum displayed high antifungal activity for compounds with an unsubstituted aromatic ring A, e.g. pinosylvin, but only weak effects for the higher substituted stilbostemin G and stemanthrenes A-C. Similar results were obtained by germ tube inhibition of five microfungi using 2-fold serial broth dilutions determined by a microplate reader. Because of weak inhibition and chemical instability of stemanthrenes, no EC(50) and EC(90) values could be calculated.


