EmetineCAS# 483-18-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 483-18-1 | SDF | Download SDF |
| PubChem ID | 10219.0 | Appearance | Powder |
| Formula | C29H40N2O4 | M.Wt | 480.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
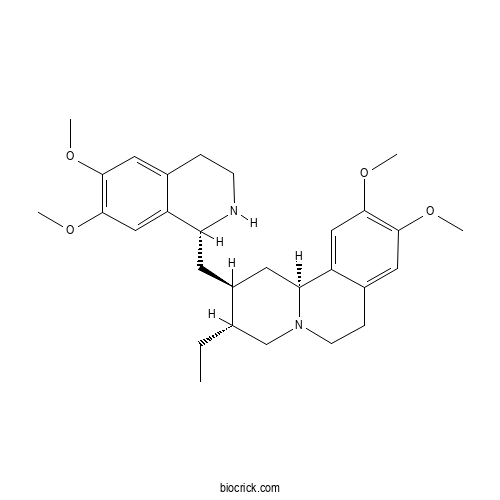
| Chemical Name | (2S,3R,11bS)-2-[[(1R)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolin-1-yl]methyl]-3-ethyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1H-benzo[a]quinolizine | ||
| SMILES | CCC1CN2CCC3=CC(=C(C=C3C2CC1CC4C5=CC(=C(C=C5CCN4)OC)OC)OC)OC | ||
| Standard InChIKey | AUVVAXYIELKVAI-CKBKHPSWSA-N | ||
| Standard InChI | InChI=1S/C29H40N2O4/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24/h13-16,18,21,24-25,30H,6-12,17H2,1-5H3/t18-,21-,24+,25-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Emetine Dilution Calculator

Emetine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0806 mL | 10.4028 mL | 20.8056 mL | 41.6112 mL | 52.014 mL |
| 5 mM | 0.4161 mL | 2.0806 mL | 4.1611 mL | 8.3222 mL | 10.4028 mL |
| 10 mM | 0.2081 mL | 1.0403 mL | 2.0806 mL | 4.1611 mL | 5.2014 mL |
| 50 mM | 0.0416 mL | 0.2081 mL | 0.4161 mL | 0.8322 mL | 1.0403 mL |
| 100 mM | 0.0208 mL | 0.104 mL | 0.2081 mL | 0.4161 mL | 0.5201 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Yuanhuadine
Catalog No.:BCX0748
CAS No.:76402-66-9
- Kuwanon B
Catalog No.:BCX0747
CAS No.:62949-78-4
- guan-fu base H
Catalog No.:BCX0746
CAS No.:4758-99-0
- Saikosaponin K
Catalog No.:BCX0745
CAS No.:405229-61-0
- Astragenol
Catalog No.:BCX0744
CAS No.:86541-79-9
- Yuanhuacine
Catalog No.:BCX0743
CAS No.:60195-70-2
- Taxifolin 7-O-β-D-glucoside
Catalog No.:BCX0742
CAS No.:14292-40-1
- Edgeworoside C
Catalog No.:BCX0741
CAS No.:126221-40-7
- Lentinan
Catalog No.:BCX0740
CAS No.:37339-90-5
- 6-Methoxyldihydrochelerythrine
Catalog No.:BCX0739
CAS No.:21080-31-9
- (-)-Myrtenal
Catalog No.:BCX0738
CAS No.:18486-69-6
- Fructo-oligosaccharide DP13/GF12
Catalog No.:BCX0737
CAS No.:137405-37-9
- Epipinoresinol-4-O-glucopyranoside
Catalog No.:BCX0750
CAS No.:24404-49-7
- Cyaonoside A
Catalog No.:BCX0751
CAS No.:110081-91-9
- Epimedin I
Catalog No.:BCX0752
CAS No.:205445-00-7
- Kuwanon T
Catalog No.:BCX0753
CAS No.:100187-66-4
- luteolin-7-O-gentiobiside
Catalog No.:BCX0754
CAS No.:70855-41-3
- Dihydroferulic acid
Catalog No.:BCX0755
CAS No.:1135-23-5
- Ergothioneine
Catalog No.:BCX0756
CAS No.:497-30-3
- Anemarrhenasaponin A2
Catalog No.:BCX0757
CAS No.:117210-12-5
- Mogroside VI A
Catalog No.:BCX0758
CAS No.:2146088-13-1
- Pulchinenoside E4
Catalog No.:BCX0759
CAS No.:1415553-83-1
- Mogroside VI B
Catalog No.:BCX0760
CAS No.:2149606-17-5
- Oleuropeinic acid
Catalog No.:BCX0761
CAS No.:96382-90-0
Emetine induces oxidative stress, cell differentiation and NF-kappaB inhibition, suppressing AML stem/progenitor cells.[Pubmed:38684672]
Cell Death Discov. 2024 Apr 29;10(1):201.
Acute myeloid leukemia (AML) is a fatal malignancy of the blood and bone marrow. Leukemic stem cells (LSCs) are a rare subset of leukemic cells that promote the development and progression of AML, and eradication of LSCs is critical for effective control of this disease. Emetine is an FDA-approved antiparasitic drug with antitumor properties; however, little is known about its potential against LSCs. Herein, we explored the antileukemic potential of Emetine, focusing on its effects on AML stem/progenitor cells. Emetine exhibited potent cytotoxic activity both in hematologic and solid cancer cells and induced AML cell differentiation. Emetine also inhibited AML stem/progenitor cells, as evidenced by decreased expression of CD34, CD97, CD99, and CD123 in KG-1a cells, indicating anti-AML stem/progenitor cell activities. The administration of Emetine at a dosage of 10 mg/kg for two weeks showed no significant toxicity and significantly reduced xenograft leukemic growth in vivo. NF-kappaB activation was reduced in Emetine-treated KG-1a cells, as shown by reduced phospho-NF-kappaB p65 (S529) and nuclear NF-kappaB p65. DNA fragmentation, YO-PRO-1 staining, mitochondrial depolarization and increased levels of active caspase-3 and cleaved PARP (Asp214) were detected in Emetine-treated KG-1a cells. Moreover, treatment with the pancaspase inhibitor Z-VAD(OMe)-FMK partially prevented the apoptotic cell death induced by Emetine. Emetine treatment also increased cellular and mitochondrial reactive oxygen species, and Emetine-induced apoptosis in KG-1a cells was partially prevented by the antioxidant N-acetylcysteine, indicating that Emetine induces apoptosis, at least in part, by inducing oxidative stress. Overall, these studies indicate that Emetine is a novel potential anti-AML agent with promising activity against stem/progenitor cells, encouraging the development of further studies aimed at its clinical application.
Antioxidant potential of alkaloids and polyphenols of Viola canescens wall using in vitro and in silico approaches.[Pubmed:38680459]
Front Chem. 2024 Apr 12;12:1379463.
Background: V. canescens Wall, a plant renowned for its ethno-medical properties, was investigated in this study for its antioxidant potential based on its wide therapeutic applications in traditional healthcare systems. The study aimed to assess the antioxidant potential of the plant extract/fractions and to predict the active phytochemicals using computational techniques. Methods: Five fractions were obtained from the crude methanolic extract of Viola canescens, and six concentrations (25, 50, 75, 100, 125, and 150 mug/mL) were prepared for each fraction. The antioxidant activity of these fractions was evaluated using the Tetraoxomolybdate (VI) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. In-silico docking studies and molecular dynamic simulations were conducted to further elucidate the molecular interactions underlying the antioxidant activity. Results: The aqueous extract of V. canescens exhibited significant antioxidant and free radical scavenging activity against DPPH. Additionally, the crude flavonoid extract demonstrated moderate activity with IC(50) value of 57.863 mug/mL, indicating potent inhibition of cell growth. In-silico docking studies revealed a strong interaction between Emetine and the aromatase protein, suggesting its potential as an antioxidant. Conclusion: The study findings highlight the antioxidant potential of V. canescens extract, indicating its suitability as a source of natural antioxidants. These results suggest its potential application in pharmaceutical preparations aimed at harnessing antioxidant properties for therapeutic purposes.
Entamoeba histolytica: In Silico and In Vitro Oligomerization of EhHSTF5 Enhances Its Binding to the HSE of the EhPgp5 Gene Promoter.[Pubmed:38673804]
Int J Mol Sci. 2024 Apr 11;25(8):4218.
Throughout its lifecycle, Entamoeba histolytica encounters a variety of stressful conditions. This parasite possesses Heat Shock Response Elements (HSEs) which are crucial for regulating the expression of various genes, aiding in its adaptation and survival. These HSEs are regulated by Heat Shock Transcription Factors (EhHSTFs). Our research has identified seven such factors in the parasite, designated as EhHSTF1 through to EhHSTF7. Significantly, under heat shock conditions and in the presence of the antiamoebic compound Emetine, EhHSTF5, EhHSTF6, and EhHSTF7 show overexpression, highlighting their essential role in gene response to these stressors. Currently, only EhHSTF7 has been confirmed to recognize the HSE as a promoter of the EhPgp5 gene (HSE_EhPgp5), leaving the binding potential of the other EhHSTFs to HSEs yet to be explored. Consequently, our study aimed to examine, both in vitro and in silico, the oligomerization, and binding capabilities of the recombinant EhHSTF5 protein (rEhHSTF5) to HSE_EhPgp5. The in vitro results indicate that the oligomerization of rEhHSTF5 is concentration-dependent, with its dimeric conformation showing a higher affinity for HSE_EhPgp5 than its monomeric state. In silico analysis suggests that the alpha 3 alpha-helix (alpha3-helix) of the DNA-binding domain (DBD5) of EhHSTF5 is crucial in binding to the major groove of HSE, primarily through hydrogen bonding and salt-bridge interactions. In summary, our results highlight the importance of oligomerization in enhancing the affinity of rEhHSTF5 for HSE_EhPgp5 and demonstrate its ability to specifically recognize structural motifs within HSE_EhPgp5. These insights significantly contribute to our understanding of one of the potential molecular mechanisms employed by this parasite to efficiently respond to various stressors, thereby enabling successful adaptation and survival within its host environment.
A glimpse into Giardia lamblia unique translational machinery.[Pubmed:38579678]
Structure. 2024 Apr 4;32(4):377-379.
Eiler et al. used cryo-electron microscopy to determine a 2.49 A resolution structure of Giardia lamblia 80S ribosome bound to tRNA, mRNA, and the anti-protozoal drug Emetine. The structure reveals some critical aspects of translation in G. lamblia, including the lack of ribosomal protein RACK1, and how Emetine blocks translation by interacting with both the ribosome and mRNA.
Exploring the molecular mechanisms of asthma across multiple datasets.[Pubmed:38489401]
Ann Med. 2024 Dec;56(1):2258926.
BACKGROUND: Asthma, a prevalent chronic respiratory disorder, remains enigmatic, notwithstanding considerable advancements in our comprehension. Continuous efforts are crucial for discovering novel molecular targets and gaining a comprehensive understanding of its pathogenesis. MATERIALS AND METHODS: In this study, we analyzed gene expression data from 212 individuals, including asthma patients and healthy controls, to identify 267 differentially expressed genes, among which C1orf64 and C7orf26 emerged as potential key genes in asthma pathogenesis. Various bioinformatics tools, including differential gene expression analysis, pathway enrichment, drug target prediction, and single-cell analysis, were employed to explore the potential roles of the genes. RESULTS: Quantitative PCR demonstrated differential expression of C1orf64 and C7orf26 in the asthmatic airway epithelial tissue, implying their potential involvement in asthma pathogenesis. GSEA enrichment analysis revealed significant enrichment of these genes in signaling pathways associated with asthma progression, such as ABC transporters, cell cycle, CAMs, DNA replication, and the Notch signaling pathway. Drug target prediction, based on upregulated and downregulated differential expression, highlighted potential asthma treatments, including Tyrphostin-AG-126, Cephalin, Verrucarin-a, and Emetine. The selection of these drugs was based on their significance in the analysis and their established anti-inflammatory and antiviral invasion properties. Utilizing Seurat and Celldex packages for single-cell sequencing analysis unveiled disease-specific gene expression patterns and cell types. Expression of C1orf64 and C7orf26 in T cells, NK cells, and B cells, instrumental in promoting hallmark features of asthma, was observed, suggesting their potential influence on asthma development and progression. CONCLUSION: This study uncovers novel genetic aspects of asthma, highlighting potential therapeutic pathways. It exemplifies the power of integrative bioinformatics in decoding complex disease patterns. However, these findings require further validation, and the precise roles of C1orf64 and C7orf26 in asthma warrant additional investigation to validate their therapeutic potential.
A ferroptosis-related ceRNA network for investigating the molecular mechanisms and the treatment of neonatal hypoxic-ischemic encephalopathy.[Pubmed:38323182]
Transl Pediatr. 2024 Jan 29;13(1):119-136.
BACKGROUND: Neonatal hypoxic-ischemic brain damage (HIBD) is a clinical syndrome causing brain injury in newborns with obscure etiology. Increasing evidence suggests that ferroptosis plays a role in HIBD. This study aimed to clarify the key ferroptosis-related genes (FRGs) of HIBD, construct a long non-coding RNA-microRNA-messenger RNA (lncRNA-miRNA-mRNA) network, and further investigate the pathogenesis of HIBD. METHODS: Gene expression data were downloaded from the Gene Expression Omnibus and FerrDb databases. The differentially expressed lncRNAs and FRGs were screened, and the related miRNAs and mRNAs were predicted. The obtained mRNA was intersected with the differentially expressed FRGs (DE-FRGs) to identify the key DE-FRGs. Cell-type Identification by Estimating Relative Subsets of RNA Transcripts method was applied to analyze the immune cell infiltration level and the relationship between key genes and immune cells. RESULTS: Gene differential expression analysis revealed that 1,178 lncRNAs, 207 miRNAs, and 647 mRNAs were differentially expressed in the blood of HIBD patients in comparison to healthy controls. The correlations of the lncRNAs, miRNAs, and mRNAs lead to the establishment of a competing endogenous RNA (ceRNA) network associated with ferroptosis in HIBD. Further validation using an external dataset and quantitative real-time polymerase chain reaction (PCR) analysis of brain tissues from hypoxic-ischemic encephalopathy rats confirmed the expression patterns of three key genes, including HMOX1, MYCN, and QSOX1. Meanwhile, the three key genes were closely correlated with the infiltration of multiple immune cells and might affect the function of HIBD regulatory genes such as CPT2 and GCK. In addition, drug prediction suggested that four drugs, including cephaeline, Emetine, mestranol, and sulmazole, might alleviate HIBD. CONCLUSIONS: Our study established a ceRNA network, identified three key genes, and predicted four drugs that are associated with ferroptosis in HIBD, which provides new ideas for the investigation of the disease mechanisms and might facilitate the diagnosis and treatment of the disease.
Molecular docking as a tool for the discovery of novel insight about the role of acid sphingomyelinase inhibitors in SARS- CoV-2 infectivity.[Pubmed:38321448]
BMC Public Health. 2024 Feb 6;24(1):395.
Recently, COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants, caused > 6 million deaths. Symptoms included respiratory strain and complications, leading to severe pneumonia. SARS-CoV-2 attaches to the ACE-2 receptor of the host cell membrane to enter. Targeting the SARS-CoV-2 entry may effectively inhibit infection. Acid sphingomyelinase (ASMase) is a lysosomal protein that catalyzes the conversion of sphingolipid (sphingomyelin) to ceramide. Ceramide molecules aggregate/assemble on the plasma membrane to form "platforms" that facilitate the viral intake into the cell. Impairing the ASMase activity will eventually disrupt viral entry into the cell. In this review, we identified the metabolism of sphingolipids, sphingolipids' role in cell signal transduction cascades, and viral infection mechanisms. Also, we outlined ASMase structure and underlying mechanisms inhibiting viral entry 40 with the aid of inhibitors of acid sphingomyelinase (FIASMAs). In silico molecular docking analyses of FIASMAs with inhibitors revealed that dilazep (S = - 12.58 kcal/mol), Emetine (S = - 11.65 kcal/mol), pimozide (S = - 11.29 kcal/mol), carvedilol (S = - 11.28 kcal/mol), mebeverine (S = - 11.14 kcal/mol), cepharanthine (S = - 11.06 kcal/mol), hydroxyzin (S = - 10.96 kcal/mol), astemizole (S = - 10.81 kcal/mol), sertindole (S = - 10.55 kcal/mol), and bepridil (S = - 10.47 kcal/mol) have higher inhibition activity than the candidate drug amiodarone (S = - 10.43 kcal/mol), making them better options for inhibition.
Insight into the Natural Biomolecules (BMs): Promising Candidates as Zika Virus Inhibitors.[Pubmed:38318833]
Infect Disord Drug Targets. 2024 Feb 2.
Zika virus (ZIKV) is among the relatively new infectious disease threats that include SARS-CoV2, coronavirus, monkeypox (Mpox) virus, etc. ZIKV has been reported to cause severe health risks to the fetus. To date, satisfactory treatment is still not available for the treatment of ZIKV infection. This review examines the last five years of work using natural biomolecules (BMs) to counteract the ZIKV through virtual screening and in vitro investigations. Virtual screening has identified doramectin, pinocembrin, hesperidins, epigallocatechin gallate, pedalitin, and quercetin as potentially active versus ZIKV infection. In vitro, testing has shown that nordihydroguaiaretic acid, mefloquine, isoquercitrin, glycyrrhetinic acid, patentiflorin-A, rottlerin, and harringtonine can reduce ZIKV infections in cell lines. However, in vivo, testing is limited, fortunately, Emetine, rottlerin, patentiflorin-A, and lycorine have shown in vivo anti- ZIKV potential. This review focuses on natural biomolecules that show a particularly high selective index (>10). There is limited in vivo and clinical trial data for natural BMs, which needs to be an active area of investigation. This review aims to compile the known reference data and discuss the barriers associated with discovering and using natural BM agents to control ZIKV infection.
Puromycin reveals a distinct conformation of neuronal ribosomes.[Pubmed:38315848]
Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2306993121.
Puromycin is covalently added to the nascent chain of proteins by the peptidyl transferase activity of the ribosome and the dissociation of the puromycylated peptide typically follows this event. It was postulated that blocking the translocation of the ribosome with Emetine could retain the puromycylated peptide on the ribosome, but evidence against this has recently been published [Hobson et al., Elife 9, e60048 (2020); and Enam et al., Elife 9, e60303 (2020)]. In neurons, puromycylated nascent chains remain in the ribosome even in the absence of Emetine, yet direct evidence for this has been lacking. Using biochemistry and cryoelectron microscopy, we show that the puromycylated peptides remain in the ribosome exit channel in the large subunit in a subset of neuronal ribosomes stalled in the hybrid state. These results validate previous experiments to localize stalled polysomes in neurons and provide insight into how neuronal ribosomes are stalled. Moreover, in these hybrid-state neuronal ribosomes, anisomycin, which usually blocks puromycylation, competes poorly with puromycin in the puromycylation reaction, allowing a simple assay to determine the proportion of nascent chains that are stalled in this state. In early hippocampal neuronal cultures, over 50% of all nascent peptides are found in these stalled polysomes. These results provide insights into the stalling mechanisms of neuronal ribosomes and suggest that puromycylated peptides can be used to reveal subcellular sites of hybrid-state stalled ribosomes in neurons.
MXSGD alleviates CsA-induced hypoimmunity lung injury by regulating microflora metabolism.[Pubmed:38259457]
Front Immunol. 2024 Jan 8;14:1298416.
CONTEXT: Ma Xing Shi Gan Decoction (MXSGD) is a traditional remedy for treating lung injuries that was developed by the Typhoid and Fever School of Pharmaceutical Biology. It has antitussive and expectorant effects, anti-inflammatory, antiviral, regulates the body's immunity, etc. AIM: The aim of this study is to investigate whether MXSGD can ameliorate cyclosporine A (CsA)-induced hypoimmunity lung injury by regulating microflora metabolism. Methods: Establishment of a model for CsA-induced hypoimmunity lung injury. Using 16S rRNA high-throughput sequencing and LC-MS, the effects of MXSGD on gut flora and lung tissue microecology of mice with CsA-induced hypoimmunity were investigated. RESULTS: MXSGD was able to preserve lung tissue morphology and structure, reduce serum inflammatory marker expression and protect against CsA-induced lung tissue damage. Compared to the model, MXSGD increased beneficial gut bacteria: Eubacterium ventriosum group and Eubacterium nodatum group; decreased intestinal pathogens: Rikenellaceae RC9 intestinal group; reduced the abundance of Chryseobacterium and Acinetobacter, promoted the production of Lactobacillus and Streptococcus, and then promoted the lung flora to produce short-chain fatty acids. MXSGD was able to enhance the expression of serum metabolites such as Americine, 2-hydroxyhexadecanoylcarnitine, Emetine, All-trans-decaprenyl diphosphate, Biliverdin-IX-alpha, Hordatin A and N-demethyl mifepristone in the CsA-induced hypoimmunity lung injury model. CONCLUSION: MXSGD can restore gut and lung microbiota diversity and serum metabolite changes to inhibit inflammation, ameliorate CsA-induced hypoimmunity lung injury.
The Giardia lamblia ribosome structure reveals divergence in several biological pathways and the mode of emetine function.[Pubmed:38242118]
Structure. 2024 Apr 4;32(4):400-410.e4.
Giardia lamblia is a deeply branching protist and a human pathogen. Its unusual biology presents the opportunity to explore conserved and fundamental molecular mechanisms. We determined the structure of the G. lamblia 80S ribosome bound to tRNA, mRNA, and the antibiotic Emetine by cryo-electron microscopy, to an overall resolution of 2.49 A. The structure reveals rapidly evolving protein and nucleotide regions, differences in the peptide exit tunnel, and likely altered ribosome quality control pathways. Examination of translation initiation factor binding sites suggests these interactions are conserved despite a divergent initiation mechanism. Highlighting the potential of G. lamblia to resolve conserved biological principles; our structure reveals the interactions of the translation inhibitor Emetine with the ribosome and mRNA, thus providing insight into the mechanism of action for this widely used antibiotic. Our work defines key questions in G. lamblia and motivates future experiments to explore the diversity of eukaryotic gene regulation.
Mechanisms of antiviral action and toxicities of ipecac alkaloids: Emetine and dehydroemetine exhibit anti-coronaviral activities at non-cardiotoxic concentrations.[Pubmed:38228190]
Virus Res. 2024 Mar;341:199322.
The emergence of highly infectious pathogens with their potential for triggering global pandemics necessitate the development of effective treatment strategies, including broad-spectrum antiviral therapies to safeguard human health. This study investigates the antiviral activity of Emetine, dehydroEmetine (DHE), and congeneric compounds against SARS-CoV-2 and HCoV-OC43, and evaluates their impact on the host cell. Concurrently, we assess the potential cardiotoxicity of these ipecac alkaloids. Significantly, our data reveal that Emetine and the (-)-R,S isomer of 2,3-dehydroEmetine (designated in this paper as DHE4) reduce viral growth at nanomolar concentrations (i.e., IC(50) approximately 50-100 nM), paralleling those required for inhibition of protein synthesis, while calcium channel blocking activity occurs at elevated concentrations (i.e., IC(50) approximately 40-60 microM). Our findings suggest that the antiviral mechanisms primarily involve disruption of host cell protein synthesis and is demonstrably stereoisomer specific. The prospect of a therapeutic window in which Emetine or DHE4 inhibit viral propagation without cardiotoxicity renders these alkaloids viable candidates in strategies worthy of clinical investigation.
Oral squamous cell carcinoma cancer stem cells have different drug sensitive to pharmacological NFkappaB and histone deacetylation inhibition.[Pubmed:38187064]
Am J Cancer Res. 2023 Dec 15;13(12):6038-6050. eCollection 2023.
Despite many progresses in the development of new systemic therapies for oral squamous cell carcinoma (OSCC), the five-year survival rate of OSCC is low. The traditional chemotherapies approach (cisplatin - CDDP) shows some limitations like drug toxicity, limited efficacy, and drug resistance. Promising studies suggested OSCC cancer stem cells (CSC) presented resistance to CDDP. We have previously studied many targets, and we extensively showed the efficacy of the NFkappaB signaling and the role of histones acetylation, on different malignant tumors, including adenoid cystic carcinoma and mucoepidermoid carcinoma, but until then the effects of the NFkB inhibitor and histone deacetylase (HDAC) inhibitor on the biology of OSCC were not evaluated. Here we assessed the pharmacological inhibitor of NFkappaB Emetine and HDAC inhibitor SAHA on the behavior of CSC derived from OSCC. Our data suggested that CDDP administration resulted in reduced viability of bulk OSCC cells and increased CSC. A single and isolated shot of Emetine and SAHA were able to disrupt CSC by inhibiting the NFkappaB pathway and increasing the histone acetylation levels, respectively. Further, the combined administration of Emetine and SAHA presented the same CSC disruption as seen in Emetine alone.
Repurposed drugs in combinations exert additive anti-chikungunya virus activity: an in-vitro study.[Pubmed:38178163]
Virol J. 2024 Jan 4;21(1):5.
Chikungunya virus (CHIKV) infection causes chikungunya, a viral disease that currently has no specific antiviral treatment. Several repurposed drug candidates have been investigated for the treatment of the disease. In order to improve the efficacy of the known drugs, combining drugs for treatment is a promising approach. The current study was undertaken to explore the antiviral activity of a combination of repurposed drugs that were reported to have anti-CHIKV activity. We explored the effect of different combinations of six effective drugs (2-fluoroadenine, Emetine, lomibuvir, enalaprilat, metyrapone and resveratrol) at their non-toxic concentrations against CHIKV under post infection treatment conditions in Vero cells. Focus-forming unit assay, real time RT-PCR, immunofluorescence assay, and western blot were used to determine the virus titre. The results revealed that the combination of 2-fluoroadenine with either metyrapone or Emetine or enalaprilat exerted inhibitory activity against CHIKV under post-infection treatment conditions. The effect of these drug combinations was additive in nature compared to the effect of the individual drugs. The results suggest an additive anti-viral effect of these drug combinations against CHIKV. The findings could serve as an outline for the development of an innovative therapeutic approach in the future to treat CHIKV-infected patients.


