YuanhuacineCAS# 60195-70-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60195-70-2 | SDF | Download SDF |
| PubChem ID | 146159319.0 | Appearance | Powder |
| Formula | C37H44O10 | M.Wt | 648.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
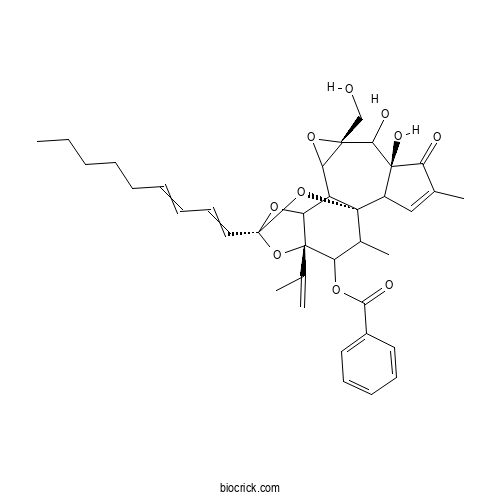
| Chemical Name | [(1R,6S,8R,14S,16S)-6,7-dihydroxy-8-(hydroxymethyl)-4,18-dimethyl-14-nona-1,3-dienyl-5-oxo-16-prop-1-en-2-yl-9,13,15,19-tetraoxahexacyclo[12.4.1.01,11.02,6.08,10.012,16]nonadec-3-en-17-yl] benzoate | ||
| SMILES | CCCCCC=CC=CC12OC3C4C5C(O5)(C(C6(C(C4(O1)C(C(C3(O2)C(=C)C)OC(=O)C7=CC=CC=C7)C)C=C(C6=O)C)O)O)CO | ||
| Standard InChIKey | CGSGRJNIABXQJQ-ZQLWRUAFSA-N | ||
| Standard InChI | InChI=1S/C37H44O10/c1-6-7-8-9-10-11-15-18-34-45-30-26-29-33(20-38,44-29)32(41)35(42)25(19-22(4)27(35)39)37(26,47-34)23(5)28(36(30,46-34)21(2)3)43-31(40)24-16-13-12-14-17-24/h10-19,23,25-26,28-30,32,38,41-42H,2,6-9,20H2,1,3-5H3/t23?,25?,26?,28?,29?,30?,32?,33-,34+,35+,36-,37-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Yuanhuacine Dilution Calculator

Yuanhuacine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5414 mL | 7.7072 mL | 15.4145 mL | 30.829 mL | 38.5362 mL |
| 5 mM | 0.3083 mL | 1.5414 mL | 3.0829 mL | 6.1658 mL | 7.7072 mL |
| 10 mM | 0.1541 mL | 0.7707 mL | 1.5414 mL | 3.0829 mL | 3.8536 mL |
| 50 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6166 mL | 0.7707 mL |
| 100 mM | 0.0154 mL | 0.0771 mL | 0.1541 mL | 0.3083 mL | 0.3854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Taxifolin 7-O-β-D-glucoside
Catalog No.:BCX0742
CAS No.:14292-40-1
- Edgeworoside C
Catalog No.:BCX0741
CAS No.:126221-40-7
- Lentinan
Catalog No.:BCX0740
CAS No.:37339-90-5
- 6-Methoxyldihydrochelerythrine
Catalog No.:BCX0739
CAS No.:21080-31-9
- (-)-Myrtenal
Catalog No.:BCX0738
CAS No.:18486-69-6
- Fructo-oligosaccharide DP13/GF12
Catalog No.:BCX0737
CAS No.:137405-37-9
- Fructo-oligosaccharide DP9/GF8
Catalog No.:BCX0736
CAS No.:143625-74-5
- Glucosinalbin
Catalog No.:BCX0735
CAS No.:19253-84-0
- Fructo-oligosaccharide DP10/GF9
Catalog No.:BCX0734
CAS No.:118150-64-4
- (9Z,12Z,15Z)-N-[(3-Methoxyphenyl)methyl]-9,12,15-octadecatrienamide
Catalog No.:BCX0733
CAS No.:883715-23-9
- Fructo-oligosaccharide DP14/GF13
Catalog No.:BCX0732
CAS No.:137405-38-0
- Maltoheptaose
Catalog No.:BCX0731
CAS No.:34620-78-5
- Astragenol
Catalog No.:BCX0744
CAS No.:86541-79-9
- Saikosaponin K
Catalog No.:BCX0745
CAS No.:405229-61-0
- guan-fu base H
Catalog No.:BCX0746
CAS No.:4758-99-0
- Kuwanon B
Catalog No.:BCX0747
CAS No.:62949-78-4
- Yuanhuadine
Catalog No.:BCX0748
CAS No.:76402-66-9
- Emetine
Catalog No.:BCX0749
CAS No.:483-18-1
- Epipinoresinol-4-O-glucopyranoside
Catalog No.:BCX0750
CAS No.:24404-49-7
- Cyaonoside A
Catalog No.:BCX0751
CAS No.:110081-91-9
- Epimedin I
Catalog No.:BCX0752
CAS No.:205445-00-7
- Kuwanon T
Catalog No.:BCX0753
CAS No.:100187-66-4
- luteolin-7-O-gentiobiside
Catalog No.:BCX0754
CAS No.:70855-41-3
- Dihydroferulic acid
Catalog No.:BCX0755
CAS No.:1135-23-5
New daphnane diterpenoidal 1,3,4-oxdiazole derivatives as potential anti-hepatoma agents: Synthesis, biological evaluation and molecular modeling studies.[Pubmed:38354501]
Bioorg Chem. 2024 Apr;145:107208.
Hepatocellular carcinoma (HCC) is a major challenge for human healthy. Daphnane-type diterpenes have attracted increasingly attention due to remarkable pharmaceutical potential including anti-HCC activity. To further develop this class of compounds as inhibitors of HCC, the daphnane diterpenoids 12-O-debenzoyl-Yuanhuacine (YHC) and 12-hydroxydaphnetoxin (YHE) were prepared by a standard chemical transformation from dried flower buds of the Daphne genkwa plant. Subsequently, 22 daphnane diterpenoidal 1,3,4-oxdiazole derivatives were rationally designed and synthesized based on YHC and YHE. The assessment of the target compound's anti-hepatocellular carcinoma activity revealed that YHC1 exhibited comparable activity to sorafenib in the Hep3B cell line, while demonstrating higher selectivity. The mechanistic investigation demonstrates that compound YHC1 induces cell cycle arrest at the G0/G1 phase, cellular senescence, apoptosis, and elevates cellular reactive oxygen species levels. Moreover, molecular docking and CETSA results confirm the interaction between YHC1 and YAP1 as well as TEAD1. Co-IP experiments further validated that YHC1 can effectively inhibit the binding of YAP1 and TEAD1. In conclusion, YHC1 selectively targets YAP1 and TEAD1, exhibiting its anti-hepatocellular carcinoma effects through the inhibition of their interaction.
Exploring the mechanism of daphne-type diterpenes against gastric cancer cells.[Pubmed:38347741]
J Asian Nat Prod Res. 2024 Feb 12:1-13.
Gastric cancer is one of the common malignant tumors. It is reported that daphne-type diterpenes have inhibitory effects on gastric cancer cells, but the mechanism is still unknown. To explore the detailed mechanism of the anticancer effect of daphne-type diterpenes, we carried out an integrated network pharmacology prediction study and selected an effective component (Yuanhuacine, YHC) for the following validation in silico and in vitro. The result showed that daphne-type diterpenes exerted an anti-tumor effect by targeting proto-oncogene tyrosine-protein kinase SRC as well as regulating the Ras/MAPK signaling pathway, which caused the apoptosis and mitochondrial damage in gastric cancer cells.
Blockage of MDM2-mediated p53 ubiquitination by yuanhuacine restrains the carcinogenesis of prostate carcinoma cells by suppressing LncRNA LINC00665.[Pubmed:36416364]
J Biochem Mol Toxicol. 2023 Mar;37(3):e23265.
Prostate cancer (PCa) is a challenging issue for men's health worldwide due to its uncontrolled proliferation and high metastatic potential. Increasing evidence has supported plant extracts and natural plant derivatives as promising antitumor therapy with less toxic side effects. Yuanhuacine is an active component isolated from Daphne genkwa and can effectively suppress the tumorigenesis of several cancers. However, its role in PCa remains unclear. In this study, Yuanhuacine dose-dependently inhibited the proliferation and induced apoptosis of PCa cells. Moreover, Yuanhuacine also restrained the invasion and migration of PCa cells. Mechanically, Yuanhuacine decreased the ubiquitination and degradation of p53 protein, and ultimately increased p53 levels, which was regulated by inhibiting the phosphorylation and total protein levels of mouse double minute 2 (MDM2). Moreover, elevation of MDM2 reversed the suppressive efficacy of Yuanhuacine in PCa cell viability, invasion, and migration. The network pharmacologic and bioinformatics analysis confirmed that MDM2 might be a common target of D. genkwa and LINC00665. Furthermore, Yuanhuacine inhibited LINC00665 expression. Upregulation of LINC00665 reversed Yuanhuacine-mediated inhibition in MDM2 protein expression and suppressed p53 levels by enhancing its ubiquitination in Yuanhuacine-treated cells. Importantly, the inhibitory effects of Yuanhuacine on cell viability and metastatic potential were offset after LINC00665 elevation. Together, the current findings highlight that Yuanhuacine may possess tumor-suppressive efficacy by inhibiting LINC00665-mediated MDM2/p53 ubiquitination signaling. Therefore, this study indicates that Yuanhuacine may be a promising candidate for the treatment of PCa.
Metabolism, pharmacokinetics, and bioavailability of yuanhuacine in rat using LC-MS.[Pubmed:36316300]
Biomed Chromatogr. 2023 Feb;37(2):e5540.
Yuanhuacine is a Daphne-type diterpene ortho-ester and is one of the main active ingredients of genkwa flos. Anticancer activity of Yuanhuacine has been well investigated in various tumor cells and animal models, but information on metabolism and pharmacokinetics is limited. The aims of the present study were to investigate the metabolic and pharmacokinetic profiles of Yuanhuacine in rat. The metabolic profile of Yuanhuacine was obtained from rat plasma, urine, and feces using ultra-high-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. A total of seven metabolites were detected, and the proposed metabolic pathways involved oxidation and glucuronidation. A simple and sensitive ultra-high-performance liquid chromatography-tandem mass spectrometry method was developed for the determination of Yuanhuacine in rat plasma. The linear range of Yuanhuacine was 1-1000 ng/ml (R(2) = 0.998). The intra- and inter-precision (coefficient of variation %) of the assay was 3.86-6.18% and 2.65-5.75%, respectively, and the intra- and inter-accuracy (relative error %) was -3.83-4.77% and -3.03-5.11%, respectively. The extraction recovery, matrix effect, stability, and incurred sample reanalysis of Yuanhuacine were within acceptable levels. The established method was validated and successfully applied to the preclinical pharmacokinetic study of Yuanhuacine. The absolute oral bioavailability of Yuanhuacine was calculated as 1.14%, and it reached the maximum plasma concentration of 28.21 +/- 2.79 ng/ml in rat plasma at 2 h in the oral dosing group. The apparent volume of distribution of intravenous and intragastric administrations was 26.07 +/- 6.45 and 21.83 +/- 3.54 L/kg, respectively. The half-life of elimination of Yuanhuacine was 9.64 +/- 1.53 h in the intravenous dosing group.
HPLC-Based Purification and Isolation of Potent Anti-HIV and Latency Reversing Daphnane Diterpenes from the Medicinal Plant Gnidia sericocephala (Thymelaeaceae).[Pubmed:35891417]
Viruses. 2022 Jun 30;14(7):1437.
Despite the success of combination antiretroviral therapy (cART), HIV persists in low- and middle-income countries (LMIC) due to emerging drug resistance and insufficient drug accessibility. Furthermore, cART does not target latently-infected CD4+ T cells, which represent a major barrier to HIV eradication. The "shock and kill" therapeutic approach aims to reactivate provirus expression in latently-infected cells in the presence of cART and target virus-expressing cells for elimination. An attractive therapeutic prototype in LMICs would therefore be capable of simultaneously inhibiting viral replication and inducing latency reversal. Here we report that Gnidia sericocephala, which is used by traditional health practitioners in South Africa for HIV/AIDS management to supplement cART, contains at least four daphnane-type compounds (Yuanhuacine A (1), Yuanhuacine as part of a mixture (2), yuanhuajine (3), and gniditrin (4)) that inhibit viral replication and/or reverse HIV latency. For example, 1 and 2 inhibit HIV replication in peripheral blood mononuclear cells (PBMC) by >80% at 0.08 microg/mL, while 1 further inhibits a subtype C virus in PBMC with a half-maximal effective concentration (EC50) of 0.03 microM without cytotoxicity. Both 1 and 2 also reverse HIV latency in vitro consistent with protein kinase C activation but at 16.7-fold lower concentrations than the control prostratin. Both 1 and 2 also reverse latency in primary CD4+ T cells from cART-suppressed donors with HIV similar to prostratin but at 6.7-fold lower concentrations. These results highlight G. sericocephala and components 1 and 2 as anti-HIV agents for improving cART efficacy and supporting HIV cure efforts in resource-limited regions.
Yuanhuacin and Related Anti-Inflammatory and Anticancer Daphnane Diterpenes from Genkwa Flos-An Overview.[Pubmed:35204693]
Biomolecules. 2022 Jan 23;12(2):192.
The dried flower buds of the plant Daphne genkwa Sieb. et Zucc. have been largely used in traditional Chinese medicine for the treatment of inflammatory diseases. Numerous diterpenoids have been isolated from the Genkwa Flos (yuanhua in Chinese), including a series of daphnane-type diterpene designated as yuanhuacin (YC, often improperly designated as Yuanhuacine) and analogues with a patronymic name. The series includes ten daphnane-type diterpenes: yuanhuacin, yuanhuadin (YD), yuanhuafin (YF), yuanhuagin (YG), yuanhuahin (YH), yuanhuajin (YJ), yuanhualin (YL), yuanhuamin (YM), yuanhuapin (YP), and yuanhuatin (YT). They are distinct from the rare flavonoid yuanhuanin. The series comprises several anticancer agents, such as the lead compound YC, which has revealed potent activity in vitro and in vivo against models of lung and breast cancers. The main signaling pathways implicated in the antitumor effects have been delineated. Protein kinase C is a key factor of activity for YC, but in general the molecular targets at the origin of the activity of these compounds remain little defined. Promising anticancer effects have been reported with analogues YD and YT, whereas compounds YF and YP are considered more toxic. The pharmacological activity of each compound is presented, as well as the properties of Genkwa Flos extracts. The potential toxic effects associated with the use of these compounds are also underlined.
Revealing the Toxicity-Enhancing Essence of Glycyrrhiza on Genkwa Flos Based on Ultra-high-performance Liquid Chromatography Coupled With Quadrupole-Orbitrap High-Resolution Mass Spectrometry and Self-Assembled Supramolecular Technology.[Pubmed:35004606]
Front Chem. 2021 Dec 23;9:740952.
Researchers often focus on the mechanisms of synergistic agents, a few explore drug combinations that enhance toxicity, while few have studied the internal mechanism of compatibility enhancement in chemical level. Herein, we present a comprehensive analysis based on ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS) and a self-assembled supramolecular strategy, which reveals the toxicity-enhancing essence of glycyrrhizic acid originated in licorice when combined with Genkwa Flos. Through this method, we discovered the toxicity was enhanced through the formation of a supramolecular complex from Genkwa Flos/glycyrrhizic acid. The morphology and size distribution of the self-assembled nanoparticles were characterized by scanning electron microscopy and dynamic light scattering Furthermore, a total of 58 constituents (eight diterpenoids, 35 flavonoids, five phenylpropanoids, four nucleosides, two amino acids, and four other compounds) consisted from the supramolecular complex were identified through accurate-mass measurements in full-scan MS/data-dependent MS/MS mode. Based on the hydrophobic interaction of glycyrrhizic acid with Yuanhuacine (one of main ingredients from Genkwa Flos), the supramolecular self-assembly mechanism was revealed with proton nuclear magnetic resonance ((1)H-NMR) and NOESY 2D NMR. The toxicity of Genkwa Flos and Genkwa Flos/glycyrrhizic acid supramolecular complex were compared through in vitro studies on L-02 cells using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay; and 4',6-diamidino-2-phenylindole (DAPI) staining was performed to further confirm the enhancement inhibition of Genkwa Flos/glycyrrhizic acid supramolecular complex than Genkwa Flos. This study provides fundamental scientific evidence of the formation of a self-assembled phytochemical supramolecular when Genkwa Flos and glycyrrhizic acid are combined, enabling to understand their clinical incompatibility and contraindication.
Yuanhuacine Is a Potent and Selective Inhibitor of the Basal-Like 2 Subtype of Triple Negative Breast Cancer with Immunogenic Potential.[Pubmed:34200174]
Cancers (Basel). 2021 Jun 7;13(11):2834.
The heterogeneity of triple negative breast cancer (TNBC) has led to efforts to further subtype this disease with the hope of identifying new molecular liabilities and drug targets. Furthermore, the finding that TNBC is the most inherently immunogenic type of breast cancer provides the potential for effective treatment with immune checkpoint inhibitors and immune adjuvants. Thus, we devised a dual screen to identify compounds from natural product extracts with TNBC subtype selectivity that also promote the expression of cytokines associated with antitumor immunity. These efforts led to the identification of Yuanhuacine (1) as a potent and highly selective inhibitor of the basal-like 2 (BL2) subtype of TNBC that also promoted an antitumor associated cytokine signature in immune cells. The mechanism of action of Yuanhuacine for both phenotypes depends on activation of protein kinase C (PKC), defining a novel target for the treatment of this clinical TNBC subtype. Yuanhuacine showed potent antitumor efficacy in animals bearing BL2 tumors further demonstrating that PKC could function as a potential pharmacological target for the treatment of the BL2 subtype of TNBC.
Anti-inflammatory and anti-angiogenic activities in vitro of eight diterpenes from Daphne genkwa based on hierarchical cluster and principal component analysis.[Pubmed:29680963]
J Nat Med. 2018 Jun;72(3):675-685.
Rheumatoid arthritis (RA) is one of the most prevalent chronic inflammatory and angiogenic diseases. The aim of this study was to evaluate the anti-inflammatory and anti-angiogenic activities in vitro of eight diterpenoids isolated from Daphne genkwa. LC-MS was used to identify diterpenes isolated from D. genkwa. The anti-inflammatory and anti-angiogenic activities of eight diterpenoids were evaluated on LPS-induced macrophage RAW264.7 cells and TNF-alpha-stimulated human umbilical vein endothelial cells (HUVECs) using hierarchical cluster analysis (HCA) and principal component analysis (PCA). The eight diterpenes isolated from D. genkwa were identified as yuanhuaphnin, isoYuanhuacine, 12-O-(2'E,4'E-decadienoyl)-4-hydroxyphorbol-13-acetyl, yuanhuagine, isoyuanhuadine, yuanhuadine, yuanhuaoate C and Yuanhuacine. All the eight diterpenes significantly down-regulated the excessive secretion of TNF-alpha, IL-6, IL-1beta and NO in LPS-induced RAW264.7 macrophages. However, only 12-O-(2'E,4'E-decadienoyl)-4-hydroxyphorbol-13-acetyl markedly reduced production of VEGF, MMP-3, ICAM and VCAM in TNF-alpha-stimulated HUVECs. HCA obtained 4 clusters, containing 12-O-(2'E,4'E-decadienoyl)-4-hydroxyphorbol-13-acetyl, isoYuanhuacine, isoyuanhuadine and five other compounds. PCA showed that the ranking of diterpenes sorted by efficacy from highest to lowest was 12-O-(2'E,4'E-decadienoyl)-4-hydroxyphorbol-13-acetyl, yuanhuaphnin, isoYuanhuacine, Yuanhuacine, yuanhuaoate C, yuanhuagine, isoyuanhuadine, yuanhuadine. In conclusion, eight diterpenes isolated from D. genkwa showed different levels of activity in LPS-induced RAW264.7 cells and TNF-alpha-stimulated HUVECs. The comprehensive evaluation of activity by HCA and PCA indicated that of the eight diterpenes, 12-O-(2'E,4'E-decadienoyl)-4-hydroxyphorbol-13-acetyl was the best, and can be developed as a new drug for RA therapy.
Pharmacokinetic comparisons of six components from raw and vinegar-processed Daphne genkwa aqueous extracts following oral administration in rats by employing UHPLC-MS/MS approaches.[Pubmed:29428673]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Mar 15;1079:34-40.
A sensitive UHPLC-MS/MS approach was developed and validated for the quantification of genkwanin, 3'-hydroxygenkwanin, apigenin, luteolin, Yuanhuacine and genkwadaphnin in biological samples after oral administration of raw and vinegar-processed Daphne genkwa. Liquiritin and glycyrrhetinic acid were employed as internal standards. Six components were extracted by using protein precipitation with acetonitrile. Chromatographic separation was achieved on a Waters BEH C(18) column (50 mm x 2.1 mm, 1.7 mum) by using a mobile phase composed of water (containing 0.1% formic acid) and acetonitrile. Mass spectrometric detection was conducted using multiple reaction monitoring (MRM). The intra- and inter-day precisions of the six analytes were below 4.87%, and the accuracies were within +/-5.0%. The extraction recoveries of the six constituents were determined between 97.5% and 105.4% and the matrix effects ranged from 97.3% to 103.7%. All the samples showed satisfactory precision and accuracy after various stability tests. The established approach was successfully applied to the comparative pharmacokinetic study. Compared to the raw group, the parameters of C(max) and AUC(0-t) of genkwanin, 3'-hydroxygenkwanin, apigenin and luteolin elevated remarkably (p < 0.05) after oral delivery of vinegar-processed Daphne genkwa while the parameters of C(max) and AUC(0-t) of Yuanhuacine and genkwadaphnin decreased significantly (p < 0.05). The results revealed that vinegar-processing could enhance bioavailability of genkwanin, 3'-hydroxygenkwanin, apigenin and luteolin but reduce the bioavailability of Yuanhuacine and genkwadaphnin.
How impaired efficacy happened between Gancao and Yuanhua: Compounds, targets and pathways.[Pubmed:28630457]
Sci Rep. 2017 Jun 19;7(1):3828.
As recorded in Traditional Chinese Medicine (TCM) theory, Gancao (Glycyrrhizae Radix et Rhizoma) could weaken the pharmacological effect or increase the toxicity of Yuanhua (Genkwa Flos). However, the theory has been suspected due to lack of evidence. Here, we investigate whether Gancao could weaken Yuanhua's diuretic effect, if so, which chemicals and which targets may be involved. Results showed that Yuanhua exerted diuretic effect through down-regulating renal AQP 2, without electrolyte disturbances such as K(+) loss which has been observed as side-effect of most diuretics. Gancao had no diuretic effect, but could impair Yuanhua's diuretic effect through up-regulating renal AQP 2. Glycyrrhetinic acid (GRA) in Gancao could up-regulate AQP 2 and counteract the AQP 2 regulation effect of Yuanhuacine (YHC) and Ginkwanin (GKW) in Yuanhua. Network pharmacology method suggested that YHC, GKW and GRA could bind to MEK1/FGFR1 protein and influence ERK-MAPK pathway, which was verified by Western blotting. This study supports TCM theory and reminds that more attention should be paid to the safety and efficacy problems induced by improper combination between herbs. Moreover, we suggested that promising diuretics with less side effects can be developed from Chinese Medicines such as Yuanhua.
Validation and Application of an Ultra High-Performance Liquid Chromatography Tandem Mass Spectrometry Method for Yuanhuacine Determination in Rat Plasma after Pulmonary Administration: Pharmacokinetic Evaluation of a New Drug Delivery System.[Pubmed:27999290]
Molecules. 2016 Dec 16;21(12):1733.
Yuanhuacine was found to have significant inhibitory activity against A-549 human lung cancer cells. However, there would be serious adverse toxicity effects after systemic administration of Yuanhuacine, such as by oral and intravenous ways. In order to achieve better curative effect and to alleviate the adverse toxicity effects, we tried to deliver Yuanhuacine directly into the lungs. Ultra high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) was used to detect the analyte and IS. After extraction (ether:dichloromethane = 8:1), the analyte and IS were separated on a Waters BEH-C(18) column (100 mm x 2.1 mm, 1.7 mum) under a 5 min gradient elution using a mixture of acetonitrile and 0.1% formic acid aqueous solution as mobile phase at a flow rate of 0.3 mL/min. ESI positive mode was chosen for detection. The method was fully validated for its selectivity, accuracy, precision, stability, matrix effect, and extraction recovery. This new method for Yuanhuacine concentration determination in rat plasma was reliable and could be applied for its preclinical and clinical monitoring purpose.


