Dihydroferulic acidCAS# 1135-23-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1135-23-5 | SDF | Download SDF |
| PubChem ID | 14340.0 | Appearance | Powder |
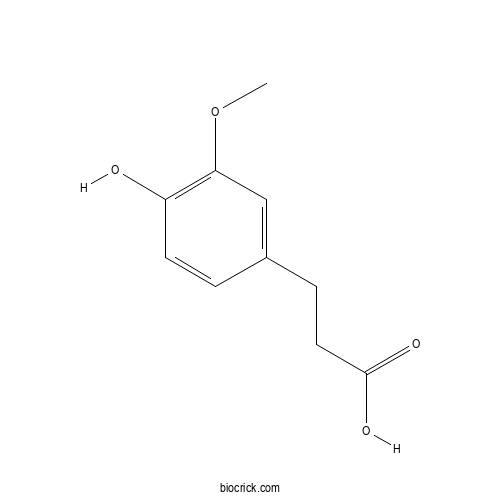
| Formula | C10H12O4 | M.Wt | 196.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(4-hydroxy-3-methoxyphenyl)propanoic acid | ||
| SMILES | COC1=C(C=CC(=C1)CCC(=O)O)O | ||
| Standard InChIKey | BOLQJTPHPSDZHR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H12O4/c1-14-9-6-7(2-4-8(9)11)3-5-10(12)13/h2,4,6,11H,3,5H2,1H3,(H,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Dihydroferulic acid Dilution Calculator

Dihydroferulic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0968 mL | 25.4842 mL | 50.9684 mL | 101.9368 mL | 127.421 mL |
| 5 mM | 1.0194 mL | 5.0968 mL | 10.1937 mL | 20.3874 mL | 25.4842 mL |
| 10 mM | 0.5097 mL | 2.5484 mL | 5.0968 mL | 10.1937 mL | 12.7421 mL |
| 50 mM | 0.1019 mL | 0.5097 mL | 1.0194 mL | 2.0387 mL | 2.5484 mL |
| 100 mM | 0.051 mL | 0.2548 mL | 0.5097 mL | 1.0194 mL | 1.2742 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- luteolin-7-O-gentiobiside
Catalog No.:BCX0754
CAS No.:70855-41-3
- Kuwanon T
Catalog No.:BCX0753
CAS No.:100187-66-4
- Epimedin I
Catalog No.:BCX0752
CAS No.:205445-00-7
- Cyaonoside A
Catalog No.:BCX0751
CAS No.:110081-91-9
- Epipinoresinol-4-O-glucopyranoside
Catalog No.:BCX0750
CAS No.:24404-49-7
- Emetine
Catalog No.:BCX0749
CAS No.:483-18-1
- Yuanhuadine
Catalog No.:BCX0748
CAS No.:76402-66-9
- Kuwanon B
Catalog No.:BCX0747
CAS No.:62949-78-4
- guan-fu base H
Catalog No.:BCX0746
CAS No.:4758-99-0
- Saikosaponin K
Catalog No.:BCX0745
CAS No.:405229-61-0
- Astragenol
Catalog No.:BCX0744
CAS No.:86541-79-9
- Yuanhuacine
Catalog No.:BCX0743
CAS No.:60195-70-2
- Ergothioneine
Catalog No.:BCX0756
CAS No.:497-30-3
- Anemarrhenasaponin A2
Catalog No.:BCX0757
CAS No.:117210-12-5
- Mogroside VI A
Catalog No.:BCX0758
CAS No.:2146088-13-1
- Pulchinenoside E4
Catalog No.:BCX0759
CAS No.:1415553-83-1
- Mogroside VI B
Catalog No.:BCX0760
CAS No.:2149606-17-5
- Oleuropeinic acid
Catalog No.:BCX0761
CAS No.:96382-90-0
- Prosapogenin D
Catalog No.:BCX0762
CAS No.:103629-72-7
- 3,4'-Dihydroxyflavone
Catalog No.:BCX0763
CAS No.:14919-49-4
- Cis-Ligupurpuroside B
Catalog No.:BCX0764
CAS No.:350588-96-4
- 6-Methylflavone
Catalog No.:BCX0765
CAS No.:29976-75-8
- Methyl Vanillate
Catalog No.:BCX0766
CAS No.:3943-74-6
- L-Amygdalin
Catalog No.:BCX0767
CAS No.:29883-16-7
Cascade Microbial Metabolism of Ferulic Acid In Vitro Fermented by the Human Fecal Inoculum.[Pubmed:38602350]
J Agric Food Chem. 2024 Apr 11.
Ferulic acid (FA), predominantly existing in most cereals, can modulate the gut microbiome, but the influences of its metabolites on the microbial population and FA-transforming microorganisms are still unclear. In this study, FA and its potential phenolic metabolites were fermented in vitro for 24 h with the human fecal inoculum. A comparable short chain fatty acid (SCFA) production trend was observed in the presence and absence of substrates, suggesting limited contribution of FA mechanism to SCFA formation. Dihydroferulic acid, 3-(3,4-dihydroxyphenyl)propionic acid, and 3-(3-hydroxyphenyl)propionic acid were ascertained to be successive metabolites of FA, by tracking the intermediate variation. FA remarkably promoted the absolute abundances of total bacteria, while different metabolites affected bacterial growth of selective genera. Specific genera were identified as quantitatively correlating to the content of FA and its metabolites. Ultimately, FA-mediated gut microbiota modulation involves both the action of metabolizing microbes and the regulation effects of metabolites on bacterial growth.
Novel insights into enzymes inhibitory responses and metabolomic profile of supercritical fluid extract from chestnut shells upon intestinal permeability.[Pubmed:38129012]
Food Res Int. 2024 Jan;175:113807.
The health benefits of chestnut (Castanea sativa) shells (CSs) have been ascribed to phytochemicals, mainly phenolic compounds. Nevertheless, an exhaustive assessment of their intestinal absorption is vital considering a possible nutraceutical application. This study evaluated the bioactivity of CSs extract prepared by Supercritical Fluid Extraction and untargeted metabolomic profile upon in-vitro intestinal permeation across a Caco-2/HT29-MTX co-culture model. The results demonstrated the neuroprotective, hypoglycemic, and hypolipidemic properties of CSs extract by inhibition of acetylcholinesterase, alpha-amylase, and lipase activities. The untargeted metabolic profiling by LC-ESI-LTQ-Orbitrap-MS unveiled almost 60 % of lipids and 30 % of phenolic compounds, with 29 metabolic pathways indicated by enrichment analysis. Among phenolics, mostly phenolic acids, flavonoids, and coumarins permeated the intestinal barrier with most metabolites arising from phase I reactions (reduction, hydrolysis, and hydrogenation) and a minor fraction from phase II reactions (methylation). The permeation rates enhanced in the following order: ellagic acid < o-coumaric acid < p-coumaric acid < ferulaldehyde Dihydroferulic acid < ferulic acid < trans-caffeic acid < trans-cinnamic acid < dihydrocaffeic acid, with better outcomes for 1000 microg/mL of extract concentration and after 4 h of permeation. Taken together, these findings sustained a considerable in-vitro intestinal absorption of phenolic compounds from CSs extract, enabling them to reach target sites and exert their biological effects.
Phenolic compound profile and gastrointestinal action of Solanaceae fruits: Species-specific differences.[Pubmed:37316011]
Food Res Int. 2023 Aug;170:112968.
In this study, the presence of phenolic compounds derived from four Solanaceae fruits (tomato, pepino, tamarillo, and goldenberry) during gastrointestinal digestion and the effect of these compounds on human gut microbiota was investigated. The results indicated that the total phenolic content of all Solanaceae fruits were increased during digestion. Furthermore, the targeted metabolic analysis identified 296 compounds, of which 71 were changed after gastrointestinal digestion in all Solanaceae fruits. Among these changed phenolic compounds, 51.3% phenolic acids and 91% flavonoids presented higher bioaccessibility in pepino and tamarillo, respectively. Moreover, higher levels of glycoside-formed phenolic acids, including Dihydroferulic acid glucoside and coumaric acid glucoside, were found in tomato fruits. In addition, tachioside showed the highest bioaccessibility in goldenberry fruits. The intake of Solanaceae fruits during the in vitro fermentation decreased the Firmicutes/Bacteroidetes ratio (F/B) compared with the control ( approximately 15-fold change on average), and goldenberry fruits showed the best effect (F/B = 2.1). Furthermore, tamarillo significantly promoted the growth of Bifidobacterium and short-chain fatty acids production. Overall, this study revealed that Solanaceae fruits had different phenolic compound profiles and health-promoting effects on the gut microbiota. It also provided relevant information to improve the consumption of Solanaceae fruits, mainly tamarillo and goldenberry fruits, due to their gut health-promoting properties, as functional foods.
Characterization of physicochemical properties, flavor volatiles and phenolic compounds of feijoa fruit varieties.[Pubmed:37044055]
Food Chem. 2023 Sep 1;419:136074.
Thirteen varieties of feijoa (Feijoa sellowiana) fruit were collected and the physical and chemical properties of feijoa peel, flesh, seed, and leaf were analyzed. Large diversities in the physicochemical characteristics and phenolic and volatile composition among various parts and between different varieties of feijoa were observed. Degrees Brix of whole fruits ranged from 10.1 (Anatoki) to 18.0 (No. 2) degrees Brix. Procyanidin B-type tetramer, procyanidin B-type dimer, and procyanidin C-type trimer had the highest concentrations in all parts and varieties of feijoa. Caffeoyl glucose, Dihydroferulic acid 4-O-glucuronide, galloyl glucose, and lariciresinol-sesquilignan were detected in feijoa fruits and leaves. A total of 105 esters, 68 terpenes, 20 alcohols, 31 hydrocarbons, 12 aldehydes, and 11 ketones were related to aromatic attributes of fruits and leaves. Early season and mid-season varieties had larger variations in the chemical properties than late-season varieties. Anatoki, Kakariki, and No.1, have the potential to be developed for attractive flavor and functional properties.
Effect of Ferulic Acid and Its Derivatives on Cold-Pressed Flaxseed Oil Oxidative Stability and Bioactive Compounds Retention during Oxidation.[Pubmed:36900605]
Foods. 2023 Mar 3;12(5):1088.
Ferulic acid (FA) is a naturally occurring phenolic antioxidant that is widely used in the food, pharmaceutical, and cosmetic industries due to its low toxicity. Its derivatives also find numerous industrial applications and may have even higher biological activity than ferulic acid. In this study, the effect of the addition of FA and its derivatives-including vanillic acid (VA), Dihydroferulic acid (DHFA), and 4-vinylguaiacol (4-VG)-on the oxidative stability of cold-pressed flaxseed oil and the degradation of bioactive compounds during oxidation was investigated. The results showed that FA and its derivatives affected the oxidative stability of flaxseed oil, but their antioxidant activity depended on the concentration (25-200 mg/100 g oil) and temperature of treatment (60-110 degrees C). Based on Rancimat test results, flaxseed oil oxidative stability predicted at 20 degrees C increased linearly with ferulic acid concentration, while its derivatives effectively prolonged the induction time at lower concentrations (50-100 mg/100 g oil). The addition of phenolic antioxidants (80 mg/100 g) generally showed a protective effect against polyunsaturated fatty acids (DHFA and 4-VG), sterols (4-VG), tocols (DHFA), squalene, and carotenoids (FA). The exception was VA, which increased the degradation of most bioactive compounds. It is believed that adding properly composed mixtures of FA and its derivatives (DHFA and 4-VG) can extend the shelf life of flaxseed oil and provide nutritional benefits.
Diverse undescribed compounds from the rhizome of Zingiber officinale Rosc. And their anti-inflammatory activity.[Pubmed:36481318]
Phytochemistry. 2023 Feb;206:113546.
Seven undescribed compounds, including a sesquiterpenoid derivative, a gamma-lactone, four gingerols, and a Dihydroferulic acid lactate were isolated from the rhizome of Zingiber officinale, and named gingerterpenoid G, gingerlactone A, zingibergingerols A-D and L-Dihydroferulic acid lactate, respectively. Zingibergingerols (+/-)-B and (+/-)-C were two pairs of enantiomers. The structures of all compounds were determined by 1D-NMR, 2D-NMR and mass spectrometry. The absolute configurations were determined by comparison of the experimental and theoretically calculated ECD curves or X-ray single-crystal diffraction. Bioassay showed that gingerterpenoid G, gingerlactone A, and zingibergingerols A and B exhibited significant anti-inflammatory activity in the model of LPS-induced RAW 264.7 cells.
Metabolite Pattern Derived from Lactiplantibacillus plantarum-Fermented Rye Foods and In Vitro Gut Fermentation Synergistically Inhibits Bacterial Growth.[Pubmed:35960594]
Mol Nutr Food Res. 2022 Nov;66(21):e2101096.
SCOPE: Fermentation improves many food characteristics using microbes, such as lactic acid bacteria (LAB). Recent studies suggest fermentation may also enhance the health properties, but mechanistic evidence is lacking. The study aims to identify a metabolite pattern reproducibly produced during sourdough and in vitro colonic fermentation of various whole-grain rye products and how it affects the growth of bacterial species of potential importance to health and disease. METHODS AND RESULTS: The study uses Lactiplantibacillus plantarum DSMZ 13890 strain, previously shown to favor rye as its substrate. Using LC-MS metabolomics, the study finds seven microbial metabolites commonly produced during the fermentations, including Dihydroferulic acid, dihydrocaffeic acid, and five amino acid metabolites, and stronger inhibition is achieved when exposing the bacteria to a mixture of the metabolites in vitro compared to individual compound exposures. CONCLUSION: The study suggests that metabolites produced by LAB may synergistically modulate the local microbial ecology, such as in the gut. This could provide new hypotheses on how fermented foods influence human health via diet-microbiota interactions.
In Vitro Intestinal Bioaccessibility and Colonic Biotransformation of Polyphenols from Mini Bell Peppers (Capsicum annuum L.).[Pubmed:35020097]
Plant Foods Hum Nutr. 2022 Mar;77(1):77-82.
To the best of our knowledge, "sweet mini bell" peppers have not been extensively investigated. In this study, we evaluated the bioaccessible phenolic compounds released during intestinal digestion and identified and quantified the microbial metabolites derived from phenolic compounds bioconversion during the in vitro colonic fermentation. A total of 66 phenolic compounds were determined. The results obtained in this study indicate that hydroxycinnamic acids (22 to 32 mg/100 g dw) and flavonoids (99 to 102 mg/100 g dw) headed by quercetin, luteolin and kaempferol glycosidic derivatives were the main bioaccessible phenolic compounds during in vitro intestinal digestion of mini bell peppers. The yellow variety contained the highest concentration of bioaccessible flavonoids (80 mg/100 g dw). For the first time in mini bell peppers, Dihydroferulic acid was detected, in the three varieties studied. 3-(4-hydroxyphenyl)propionic acid was the major metabolite found after 12-24 h fermentation of all samples (44 to 102 microM/L). Further cell culture or in vivo studies are needed to elucidate the biological activities of the phenolic compounds identified in mini bell peppers.
Three new tyrosol derivatives from Huangjing wine.[Pubmed:34842008]
J Asian Nat Prod Res. 2022 Nov;24(11):1018-1024.
Phytochemical investigation on the concentrate of Huangjing wine, resulted in the isolation of three new tyrosol derivatives 4'''-hydroxyphenethyl 2-(R)-hydroxy-3-phenylpropionate (1), 4'''-hydroxyphenethyl(4'-hydroxy-3'-methoxyphenyl)propionate (2) and 4''-hydroxyphenethyl ethyl succinate (3), together with 5 known compounds, ferulic acid (4), L-phenyllactic acid (5), hydroxytyrosol (6), Dihydroferulic acid (7), cyclo(L-Pro-D-Tyr) (8). Their structures were elucidated using spectroscopic analysis and by comparison with the literature data. All compounds displayed antioxidant effect in the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical. Among them, the new compound 2 exhibited obvious antioxidant effect, and new compounds 1 and 3 exhibited medium antioxidant effect.
Ferulic Acid Metabolites Attenuate LPS-Induced Inflammatory Response in Enterocyte-like Cells.[Pubmed:34579029]
Nutrients. 2021 Sep 10;13(9):3152.
Ferulic acid (FA) is a polyphenol pertaining to the class of hydroxycinnamic acids present in numerous foods of a plant origin. Its dietary consumption leads to the formation of several phase I and II metabolites in vivo, which represent the largest amount of ferulates in the circulation and in the intestine in comparison with FA itself. In this work, we evaluated their efficacy against the proinflammatory effects induced by lipopolysaccharide (LPS) in intestinal Caco-2 cell monolayers, as well as the mechanisms underlying their protective action. LPS-induced overexpression of proinflammatory enzymes such as inducible nitric oxide synthase (iNOS) and the consequent hyperproduction of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) were limited by physiological relevant concentrations (1 microM) of FA, its derivatives isoferulic acid (IFA) and Dihydroferulic acid (DHFA), and their glucuronidated and sulfated metabolites, which acted upstream by limiting the activation of MAPK p38 and ERK and of Akt kinase, thus decreasing the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) translocation into the nucleus. Furthermore, the compounds were found to promote the expression of Nrf2, which may have contributed to the downregulation of NF-kB activity. The overall data show that phase I/II metabolites retain the efficacy of their dietary free form in contrasting inflammatory response.
Cytotoxic, Antioxidant, and Antidiabetic Activities versus UPLC-ESI-QTOF-MS Chemical-Profile Analysis of Ipomoea aquatica Fractions.[Pubmed:34359082]
Planta Med. 2021 Oct;87(12-13):1089-1100.
Ipomoea aquatica is a common green leafy vegetable that has numerous uses in traditional medicine. This study focused on the determination of the cytotoxic, antiradical, and antidiabetic properties of various fractions of the I. aquatica methanolic extract, as well as on the tentative identification of some bioactive compounds in the same fractions. The cytotoxicity was determined by the brine shrimp lethal test. The antioxidant activities of the I. aquatica fractions were investigated through 3 assays. The antidiabetic activity (in vitro) was measured by alpha-glucosidase and alpha-amylase inhibition assays. Phytochemical qualitative analyses demonstrated the presence of alkaloids, terpenoids, phenols, and flavonoids in the ethyl acetate-methanol and methanol fractions. The total phenolic and total flavonoid contents were found to be highest in the ethyl acetate-MeOH fractions. The evaluation of the cytotoxicity showed that the hexane-dichloromethane fraction is the most toxic, while the others are moderately toxic. The antioxidant activity assays showed that the ethyl acetate-MeOH fractions are the most potent, while the alpha-glucosidase and alpha-amylase assays revealed that the hexane-dichloromethane fraction might contain a potent antidiabetic agent. Some bioactive substances in the MeOH fractions, such as salicylic acid glucoside, 1-O-sinapoyl-beta-D-glucose derivative, and Dihydroferulic acid derivative, were tentatively identified. To the best of our knowledge, this is the first report to detect and identify these compounds in this species. Based on the results of this study, it may be concluded that I. aquatica is a potent antioxidant agent and could be a good candidate as a natural antioxidant in food and therapeutics.
Ferulic Acid Derivatives and Avenanthramides Modulate Endothelial Function through Maintenance of Nitric Oxide Balance in HUVEC Cells.[Pubmed:34204635]
Nutrients. 2021 Jun 12;13(6):2026.
Wholegrain oats contain a variety of phenolic compounds thought to help maintain healthy vascular function, through the maintenance of local levels of the vasodilator nitric oxide (NO). Thus, the full molecular mechanisms involved are not yet clear. With this work we aim to understand the possible cellular mechanisms by which avenanthramides and ferulic acid derivatives, present in oats, may help maintain a healthy vascular function through the modulation of the NO pathway. Primary Human Umbilical Vein Endothelial Cells (HUVEC) were exposed to ferulic acid, isoferulic acid, hydroferulic acid, ferulic acid 4-O-glucuronide, isoferulic acid 3-O-sulfate, Dihydroferulic acid 4-O-glucuronide, avenanthramide A, avenanthramide B and avenanthramide C (1 muM) or vehicle (methanol) for 24 h. Apocynin and Nomega-Nitro-L-arginine (L-NNA) were additionally included as controls. NO and cyclic GMP (cGMP) levels, superoxide production and the activation of the Akt1/eNOS pathway were assessed. The statistical analysis was performed using one-way ANOVA followed by a Tukey post-hoc t-test. Apocynin and all phenolic compounds increased NO levels in HUVEC cells (increased DAF2-DA fluorescence and cGMP), and significantly reduced superoxide levels. Protein expression results highlighted an increase in the Akt1 activation state, and increased eNOS expression. Overall, our results indicated that the glucuronide metabolites do not enhance NO production through the Akt1/eNOS pathway, thus all compounds tested are able to reduce NO degradation through reduced superoxide formation.
Sourdough yeast-bacteria interactions can change ferulic acid metabolism during fermentation.[Pubmed:33875218]
Food Microbiol. 2021 Sep;98:103790.
The metabolism of ferulic acid (FA) was studied during fermentation with different species and strains of lactic acid bacteria (LAB) and yeasts, in synthetic sourdough medium. Yeast strains of Kazachstania humilis, Kazachstania bulderi, and Saccharomyces cerevisiae, as well as lactic acid bacteria strains of Fructilactobacillus sanfranciscensis, Lactiplantibacillus plantarum, Lactiplantibacillus xiangfangensis, Levilactobacillus hammesii, Latilactobacillus curvatus and Latilactobacillus sakei were selected from French natural sourdoughs. Fermentation in presence or absence of FA was carried out in LAB and yeasts monocultures, as well as in LAB/yeast co-cultures. Our results indicated that FA was mainly metabolized into 4-vinylguaiacol (4-VG) by S. cerevisiae strains, and into Dihydroferulic acid (DHFA) and 4-VG in the case of LAB. Interactions of LAB and yeasts led to the modification of FA metabolism, with a major formation of DHFA, even by the strains that do not produce it in monoculture. Interestingly, FA was almost completely consumed by the F. sanfranciscensis bFs17 and K. humilis yKh17 pair and converted into DHFA in 89.5 +/- 19.6% yield, while neither bFs17, nor yKh17 strains assimilated FA in monoculture.
1, 5-dicaffeoylquinic acid, an alpha-glucosidase inhibitor from the root of Dorema ammoniacum D. Don.[Pubmed:33628284]
Res Pharm Sci. 2020 Oct 19;15(5):429-436.
BACKGROUND AND PURPOSE: Dorema ammoniacum D. Don (Apiaceae family) is a perennial plant whose oleo- gum resin is used as a natural remedy for various diseases, especially chronic bronchitis, and asthma. In the present study, hydromethanolic extract of D. ammoniacum root was subjected to phytochemical analyses and alpha-glucosidase inhibitory potentials of the isolated compounds were assessed. EXPERIMENTAL APPROACH: Silica gel (normal and reversed phases) and Sephadex((R)) LH-20 column chromatographies were used for the isolation and purification of the compounds. Structures of the compounds were characterized by 1D and 2D nuclear magnetic resonance (NMR) techniques. All the isolated compounds were assessed for their in vitro alpha-glucosidase inhibitory activity in comparison with acarbose, a standard drug. FINDINGS/RESULTS: Two phloroacetophenone glycosides; echisoside (1) and pleoside (2), along with Dihydroferulic acid-4-O-beta-D-glucopyranoside (3), and beta-resorcylic acid (4), and two caffeoylquinic acid derivatives; chlorogenic acid (5) and 1, 5-dicaffeoylquinic acid (cynarin, 6) were isolated. Among the isolated compounds, the alpha-glucosidase inhibitory effect of 1,5-dicaffeoylquinic acid was found as 76.9% of the acarbose activity at 750 muM (IC(50) value of acarbose). CONCLUSION AND IMPLICATIONS: Considerable alpha-glucosidase inhibitory effect of 1,5-dicaffeoylquinic acid makes it an appropriate candidate for further studies in the development of new natural antidiabetic drugs.


