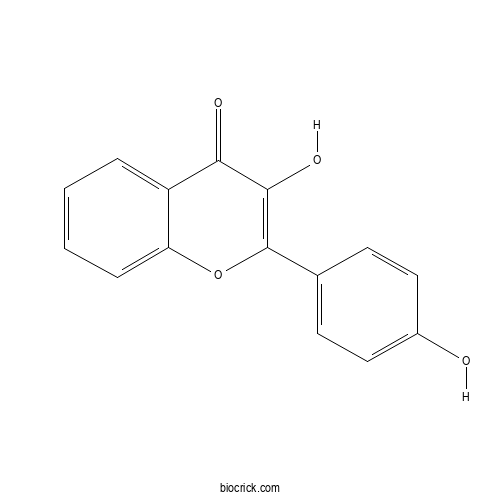
3,4'-DihydroxyflavoneCAS# 14919-49-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14919-49-4 | SDF | Download SDF |
| PubChem ID | 688715.0 | Appearance | Powder |
| Formula | C15O4H10 | M.Wt | 254.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-hydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C(=C(O2)C3=CC=C(C=C3)O)O | ||
| Standard InChIKey | GPGOCTLAUAHUQO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O4/c16-10-7-5-9(6-8-10)15-14(18)13(17)11-3-1-2-4-12(11)19-15/h1-8,16,18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3,4'-Dihydroxyflavone Dilution Calculator

3,4'-Dihydroxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9333 mL | 19.6665 mL | 39.3329 mL | 78.6658 mL | 98.3323 mL |
| 5 mM | 0.7867 mL | 3.9333 mL | 7.8666 mL | 15.7332 mL | 19.6665 mL |
| 10 mM | 0.3933 mL | 1.9666 mL | 3.9333 mL | 7.8666 mL | 9.8332 mL |
| 50 mM | 0.0787 mL | 0.3933 mL | 0.7867 mL | 1.5733 mL | 1.9666 mL |
| 100 mM | 0.0393 mL | 0.1967 mL | 0.3933 mL | 0.7867 mL | 0.9833 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Prosapogenin D
Catalog No.:BCX0762
CAS No.:103629-72-7
- Oleuropeinic acid
Catalog No.:BCX0761
CAS No.:96382-90-0
- Mogroside VI B
Catalog No.:BCX0760
CAS No.:2149606-17-5
- Pulchinenoside E4
Catalog No.:BCX0759
CAS No.:1415553-83-1
- Mogroside VI A
Catalog No.:BCX0758
CAS No.:2146088-13-1
- Anemarrhenasaponin A2
Catalog No.:BCX0757
CAS No.:117210-12-5
- Ergothioneine
Catalog No.:BCX0756
CAS No.:497-30-3
- Dihydroferulic acid
Catalog No.:BCX0755
CAS No.:1135-23-5
- luteolin-7-O-gentiobiside
Catalog No.:BCX0754
CAS No.:70855-41-3
- Kuwanon T
Catalog No.:BCX0753
CAS No.:100187-66-4
- Epimedin I
Catalog No.:BCX0752
CAS No.:205445-00-7
- Cyaonoside A
Catalog No.:BCX0751
CAS No.:110081-91-9
- Cis-Ligupurpuroside B
Catalog No.:BCX0764
CAS No.:350588-96-4
- 6-Methylflavone
Catalog No.:BCX0765
CAS No.:29976-75-8
- Methyl Vanillate
Catalog No.:BCX0766
CAS No.:3943-74-6
- L-Amygdalin
Catalog No.:BCX0767
CAS No.:29883-16-7
- 2α,6β,23-trihydroxyl oleanolic acid
Catalog No.:BCX0768
CAS No.:564-13-6
- Hirudonucleodisulfide B
Catalog No.:BCX0769
CAS No.:1072789-38-8
- Hederoside D2
Catalog No.:BCX0770
CAS No.:20853-58-1
- (Z)-9-Nonadecene
Catalog No.:BCX0771
CAS No.:51865-02-2
- 2-Hydroxycinnamicaldehyde
Catalog No.:BCX0772
CAS No.:3541-42-2
- Nardoguaianone K
Catalog No.:BCX0773
CAS No.:443128-65-2
- 2,3-dihydroxypropyl 9-octadecenoate
Catalog No.:BCX0774
CAS No.:251983-54-7
- 20-Deoxy,5-benzoyl-Ingenol
Catalog No.:BCX0775
CAS No.:54706-97-7
Predictive QSAR model confirms flavonoids in Chinese medicine can activate voltage-gated calcium (CaV) channel in osteogenesis.[Pubmed:32256687]
Chin Med. 2020 Mar 31;15:31.
BACKGROUND: Flavonoids in Chinese Medicine have been proven in animal studies that could aid in osteogenesis and bone formation. However, there is no consented mechanism for how these phytochemicals action on the bone-forming osteoblasts, and henceforth the prediction model of chemical screening for this specific biochemical function has not been established. The purpose of this study was to develop a novel selection and effective approach of flavonoids on the prediction of bone-forming ability via osteoblastic voltage-gated calcium (CaV) activation and inhibition using molecular modelling technique. METHOD: Quantitative structure-activity relationship (QSAR) in supervised maching-learning approach is applied in this study to predict the behavioral manifestations of flavonoids in the CaV channels, and developing statistical correlation between the biochemical features and the behavioral manifestations of 24 compounds (Training set: Kaempferol, Taxifolin, Daidzein, Morin, Scutellarein, Quercetin, Apigenin, Myricetin, Tamarixetin, Rutin, Genistein, 5,7,2'-Trihydroxyflavone, Baicalein, Luteolin, Galangin, Chrysin, Isorhamnetin, Naringin, 3-Methyl galangin, Resokaempferol; test set: 5-Hydroxyflavone, 3,6,4'-Trihydroxyflavone, 3,4'-Dihydroxyflavone and Naringenin). Based on statistical algorithm, QSAR provides a reasonable basis for establishing a predictive correlation model by a variety of molecular descriptors that are able to identify as well as analyse the biochemical features of flavonoids that engaged in activating or inhibiting the CaV channels for osteoblasts. RESULTS: The model has shown these flavonoids have high activating effects on CaV channel for osteogenesis. In addition, scutellarein was ranked the highest among the screened flavonoids, and other lower ranked compounds, such as daidzein, quercetin, genistein and naringin, have shown the same descending order as previous animal studies. CONCLUSION: This predictive modelling study has confirmed and validated the biochemical activity of the flavonoids in the osteoblastic CaV activation.
Anti-allergic activities of 5,7-dimethoxy-3,4'-dihydroxyflavone via inhalation in rat allergic models.[Pubmed:30707957]
Eur J Pharmacol. 2019 Apr 5;848:55-61.
Various studies have shown that flavones have several pharmacological activities including anti-allergy activities. However, the bioavailability of oral flavones is very low, and whether inhaled administration can improve efficacy in respiratory disease models is unclear. In the present study, the anti-allergic activities of inhaling 5,7-dimethoxy-3,4'-dihydroxyflavone (MHF), a synthetic flavonoid, was investigated by comparison with disodium cromoglycate (DSCG) and nedocromil sodium (NS) in rat allergic models. In an anti-DNP-IgE-induced asthmatic model, inhaled MHF dose-dependently inhibited the increase in airway resistance after antigen challenge. In an ovalbumin (OVA)-induced asthmatic model, inhaled MHF showed significant suppression of airway hyperresponsiveness; a decrease in eosinophil and neutrophil counts, IL-4, IL-5 and leukotriene D(4) in bronchoalveolar lavage fluid; a reduction in total IgE and OVA-specific IgE levels in serum; and suppression of eosinophil infiltration in lung tissue after antigen challenge. The efficacy of inhaled MHF was comparable to that of NS and DSCG. In conclusion, based on these findings, the report for the first time that that inhaled MHF may be a potential drug for the treatment of allergic asthma.
Regulation of Adipogenesis Through Differential Modulation of ROS and Kinase Signaling Pathways by 3,4'-Dihydroxyflavone Treatment.[Pubmed:27579626]
J Cell Biochem. 2017 May;118(5):1065-1077.
Studies on adipogenesis may be important for regulating human and/or animal obesity, which causes several complications such as, type II diabetes, hypertension, and cardiovascular disease, thus giving rise to increased economic burden in many countries. Previous reports revealed that various flavonoids have anti-apoptotic, antioxidant, and cell differentiation-regulating activities with a number of physiological benefits, including protection from cardiovascular disease, cancers, and oxidative stress. As we found that the hydroxylation patterns of the flavonoid B ring are known to play a critical role in their function, we screened several flavonoids containing different numbers and positions of OH substitutions in B ring for their modulatory property on adipogenesis. In this study, we revealed the anti-adipogenic activity of the naturally derived flavonoid, 3,4'-dihydroxyflavone (3,4'-DHF) in murine 3T3-L1 pre-adipocytes and equine adipose-derived stromal cells (eADSCs). We found that treatment with 3,4'-dihydroxyflavone (3,4'-DHF) led to decreased expression of adipogenic markers and lipid deposition with differential modulation of ROS and kinase signaling pathways. Regulation of ROS generation through the differential modulation of ROS-regulating gene expression was revealed to have an important role in the suppression of adipogenesis and increase of osteogenesis in eADSCs following 3,4'-DHF treatment. These results suggest that the flavonoid 3,4'-DHF can be used to regulate adipogenesis in ADSCs, which has potential therapeutic application in regenerative medicine or health care for humans and many sport or companion animals. J. Cell. Biochem. 118: 1065-1077, 2017. (c) 2016 Wiley Periodicals, Inc.


