EndoxifenCAS# 112093-28-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112093-28-4 | SDF | Download SDF |
| PubChem ID | 10090750 | Appearance | Powder |
| Formula | C25H27NO2 | M.Wt | 373.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (133.87 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
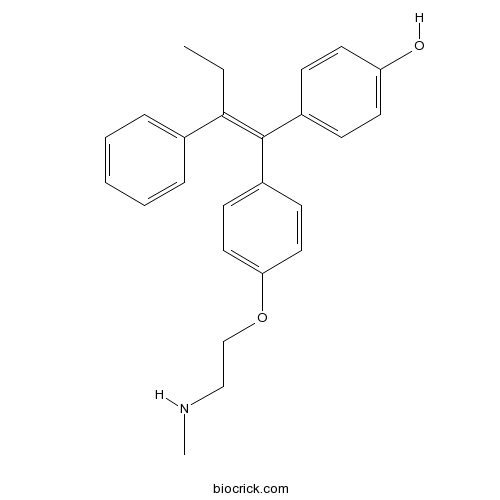
| Chemical Name | 4-[(Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol | ||
| SMILES | CCC(=C(C1=CC=C(C=C1)O)C2=CC=C(C=C2)OCCNC)C3=CC=CC=C3 | ||
| Standard InChIKey | MHJBZVSGOZTKRH-IZHYLOQSSA-N | ||
| Standard InChI | InChI=1S/C25H27NO2/c1-3-24(19-7-5-4-6-8-19)25(20-9-13-22(27)14-10-20)21-11-15-23(16-12-21)28-18-17-26-2/h4-16,26-27H,3,17-18H2,1-2H3/b25-24- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Estrogen receptor α (ERα) ligand; potent antiestrogen. Metabolite of tamoxifen. Primary metabolite responsible for the effectiveness of tamoxifen in ER-positive breast cancer. |

Endoxifen Dilution Calculator

Endoxifen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6774 mL | 13.3872 mL | 26.7745 mL | 53.549 mL | 66.9362 mL |
| 5 mM | 0.5355 mL | 2.6774 mL | 5.3549 mL | 10.7098 mL | 13.3872 mL |
| 10 mM | 0.2677 mL | 1.3387 mL | 2.6774 mL | 5.3549 mL | 6.6936 mL |
| 50 mM | 0.0535 mL | 0.2677 mL | 0.5355 mL | 1.071 mL | 1.3387 mL |
| 100 mM | 0.0268 mL | 0.1339 mL | 0.2677 mL | 0.5355 mL | 0.6694 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
- (R)-(-)-Modafinic acid
Catalog No.:BCC5157
CAS No.:112111-45-2
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
- 16-O-Methyl-14,15-didehydroisovincanol
Catalog No.:BCN1618
CAS No.:112237-71-5
- Stigmastane-3,6-diol
Catalog No.:BCN6003
CAS No.:112244-29-8
- (S)-tert-Leucinol
Catalog No.:BCN8367
CAS No.:112245-13-3
- 20(R)-Ginsenoside Rh2
Catalog No.:BCN2484
CAS No.:112246-15-8
- H-Asp(OcHex)-OH
Catalog No.:BCC2887
CAS No.:112259-66-2
Synergistic disruption of ERalpha/HER2 crosstalk by endoxifen and lapatinib in breast cancer cells.[Pubmed:27942916]
Cancer Chemother Pharmacol. 2017 Jan;79(1):117-130.
BACKGROUND: Despite decades of clinical success, tamoxifen therapy is complicated by inter-individual variability due to CYP450 polymorphism and resistance attributed to ERalpha/HER2 crosstalk. Direct administration of Endoxifen shows promise in circumventing obligatory CYP450 bioactivation while maintaining efficacy. Separately, disruption of the crosstalk using probe antagonists against ERalpha (tamoxifen) and HER2 (e.g., lapatinib) has been explored clinically. However, the efficacy of this combination may be confounded by lapatinib, a potent inactivator of CYP3A4/5 which could negate the bioactivation of tamoxifen to the active metabolite Endoxifen. Additionally, in a manner analogous to tamoxifen, Endoxifen is similarly not immune to the development of ERalpha/HER2 crosstalk that could result in resistance. Simultaneous antagonism of ERalpha and HER2 using Endoxifen and lapatinib could overcome these problems. METHODS: Metabolism studies were performed in human liver microsomes to determine the extent of inhibition of tamoxifen bioactivation by lapatinib. Synergism of Endoxifen and lapatinib was assessed using the combination index design in a panel of cell models exhibiting either a priori ERalpha/HER2 crosstalk (BT474) or acquired ERalpha/HER2 crosstalk (TAM-R and MCF-7/HER2). RESULTS: Lapatinib inhibited tamoxifen bioactivation by up to 1.8-fold. Synergistic activity was uncovered for lapatinib and Endoxifen against BT474, TAM-R and MCF-7/HER2 models of ERalpha/HER2 crosstalk. Western blot confirmed that Endoxifen and lapatinib disrupted this crosstalk. CONCLUSION: This forward-looking study extends the success of tamoxifen by exploring the effectiveness of combining the next-generation tamoxifen derivative, Endoxifen with an anti-HER2 agent to combat ERalpha/HER2 crosstalk, and at the same time provides a solution to the predicted pharmacokinetic antagonism between lapatinib and tamoxifen.
Utility of (18)F-fluoroestradiol ((18)F-FES) PET/CT imaging as a pharmacodynamic marker in patients with refractory estrogen receptor-positive solid tumors receiving Z-endoxifen therapy.[Pubmed:27872957]
Eur J Nucl Med Mol Imaging. 2017 Mar;44(3):500-508.
BACKGROUND: Z-Endoxifen is the most potent of the metabolites of tamoxifen, and has the potential to be more effective than tamoxifen because it bypasses potential drug resistance mechanisms attributable to patient variability in the expression of the hepatic microsomal enzyme CYP2D6. (18)F-FES is a positron emission tomography (PET) imaging agent which selectively binds to estrogen receptor alpha (ER-alpha) and has been used for non-invasive in vivo assessment of ER activity in tumors. This study utilizes (18)F-FES PET imaging as a pharmacodynamic biomarker in patients with ER+ tumors treated with Z-Endoxifen. METHODS: Fifteen patients were recruited from a parent therapeutic trial of Z-Endoxifen and underwent imaging with (18)F-FES PET at baseline. Eight had positive lesions on the baseline scan and underwent follow-up imaging with (18)F-FES 1-5 days post administration of Z-Endoxifen. RESULTS: Statistically significant changes (p = 0.0078) in standard uptake value (SUV)-Max were observed between the baseline and follow-up scans as early as 1 day post drug administration. CONCLUSION: F-FES PET imaging could serve as a pharmacodynamic biomarker for patients treated with ER-directed therapy.
Addressing Adherence Using Genotype-Specific PBPK Modeling-Impact of Drug Holidays on Tamoxifen and Endoxifen Plasma Levels.[Pubmed:28382001]
Front Pharmacol. 2017 Mar 14;8:67.
Introduction: Tamoxifen is one of the most common treatment opportunities for hormonal positive breast cancer. Despite its good tolerability, patients demonstrate decreasing adherence over years impacting on therapeutic success. PBPK modeling was applied to demonstrate the impact of drug holidays on plasma levels of tamoxifen and its active metabolite Endoxifen for different CYP2D6 genotypes. Materials and Methods: A virtual study with 24,000 patients was conducted in order to investigate the development of tamoxifen steady-state kinetics in patient groups of different CYP2D6 genotypes. The impact of drug holidays on steady-state kinetics was investigated assuming changing drug holiday scenarios. Results: Drug holidays in CYP2D6 extensive and intermediate metabolizers (EMs, IMs) exceeding 1 month lead to a decrease of Endoxifen steady-state trough levels below the 5th percentile of the control group. Assuming drug holidays of 1, 2, or 3 months and administering a fixed-dose combination of 20 mg tamoxifen and 3 mg Endoxifen EMs demonstrated re-established Endoxifen steady-state trough levels after 5, 8, and 9 days. IMs receiving the same fixed-dose combination demonstrated re-established Endoxifen steady-state trough levels after 7, 10, and 11 days. Discussion: The PBPK model impressively demonstrates the impact of drug holidays in different CYP2D6 genotypes on PK. Population simulation results indicate that drug holidays of more than 2 weeks cause a tremendous decrease of plasma levels despite the long half-life of tamoxifen. To improve therapeutic success, PBPK modeling allows identifying genotype-specific differences in PK following drug holidays and adequate treatment with loading doses.
Fast and Adequate Liquid Chromatography-Tandem Mass Spectrometric Determination of Z-endoxifen Serum Levels for Therapeutic Drug Monitoring.[Pubmed:28045782]
Ther Drug Monit. 2017 Apr;39(2):132-137.
BACKGROUND: Z-Endoxifen (further referred to as Endoxifen, unless stated otherwise) is proposed as the most important metabolite of tamoxifen. Patients receiving adjuvant tamoxifen treatment with Endoxifen levels below the threshold of 5.9 ng/mL may have an increased risk of breast cancer recurrence. Several factors, such as genetic polymorphisms, drug interactions, and (non)adherence, lead to large interpatient variability in Endoxifen exposure, resulting in a substantial number of patients showing subtherapeutic levels. As genotyping and phenotyping are not able to adequately predict Endoxifen exposure, therapeutic drug monitoring (TDM) seems to be the best approach for tailored tamoxifen therapy. METHODS: To support TDM services, a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry assay for the quantification of Endoxifen in human serum was developed and validated. Validation was performed according to the latest US FDA and EMA guidelines on bioanalytical method validation. RESULTS: The successfully validated serum assay quantifies Endoxifen with a linear regression calibration model (weighted 1/x) in the concentration range from 1.00 to 25.0 ng/mL. The assay was validated with an inaccuracy of +/-7.7% and an imprecision of 66. CONCLUSIONS: All validation parameters fulfilled their acceptance criteria, and the developed assay is now successfully being used to support TDM services. Thus far, 32.7% of the more than 500 determined Endoxifen serum levels were below the threshold of 5.9 ng/mL.
Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen.[Pubmed:21392396]
Breast Cancer Res. 2011 Mar 10;13(2):R27.
INTRODUCTION: We have previously demonstrated that Endoxifen is the most important tamoxifen metabolite responsible for eliciting the anti-estrogenic effects of this drug in breast cancer cells expressing estrogen receptor-alpha (ERalpha). However, the relevance of ERbeta in mediating Endoxifen action has yet to be explored. Here, we characterize the molecular actions of Endoxifen in breast cancer cells expressing ERbeta and examine its effectiveness as an anti-estrogenic agent in these cell lines. METHODS: MCF7, Hs578T and U2OS cells were stably transfected with full-length ERbeta. ERbeta protein stability, dimer formation with ERalpha and expression of known ER target genes were characterized following Endoxifen exposure. The ability of various Endoxifen concentrations to block estrogen-induced proliferation of MCF7 parental and ERbeta-expressing cells was determined. The global gene expression profiles of these two cell lines was monitored following estrogen and Endoxifen exposure and biological pathway analysis of these data sets was conducted to identify altered cellular processes. RESULTS: Our data demonstrate that Endoxifen stabilizes ERbeta protein, unlike its targeted degradation of ERalpha, and induces ERalpha/ERbeta heterodimerization in a concentration dependent manner. Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERbeta is expressed. Additionally, low concentrations of Endoxifen observed in tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERbeta, whereas much higher Endoxifen concentrations are needed when ERbeta is absent. Microarray analyses reveal substantial differences in the global gene expression profiles induced by Endoxifen at low concentrations (40 nM) when comparing MCF7 cells which express ERbeta to those that do not. These profiles implicate pathways related to cell proliferation and apoptosis in mediating Endoxifen effectiveness at these lower concentrations. CONCLUSIONS: Taken together, these data demonstrate that the presence of ERbeta enhances the sensitivity of breast cancer cells to the anti-estrogenic effects of Endoxifen likely through the molecular actions of ERalpha/beta heterodimers. These findings underscore the need to further elucidate the role of ERbeta in the biology and treatment of breast cancer and suggest that the importance of pharmacologic variation in Endoxifen concentrations may differ according to ERbeta expression.
The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells.[Pubmed:19244106]
Cancer Res. 2009 Mar 1;69(5):1722-7.
Tamoxifen has been the most important therapeutic agent for the treatment of estrogen receptor (ER)-positive breast cancer for the past three decades. Tamoxifen is extensively metabolized by cytochrome P450 enzymes, and recent in vivo studies have shown that women with genetically impaired cytochrome P450 2D6 have reduced production of Endoxifen and a higher risk of breast cancer recurrence. Despite these observations, the contribution of Endoxifen to the overall drug effectiveness of tamoxifen remains uncertain. Here, we provide novel evidence that Endoxifen is a potent antiestrogen that functions in part by targeting ERalpha for degradation by the proteasome in breast cancer cells. Additionally, we show that Endoxifen blocks ERalpha transcriptional activity and inhibits estrogen-induced breast cancer cell proliferation even in the presence of tamoxifen, N-desmethyl-tamoxifen, and 4-hydroxytamoxifen. All of the effects of Endoxifen are concentration dependent and do not occur at concentrations observed in human CYP2D6 poor metabolizers. These results support the theory that Endoxifen is the primary metabolite responsible for the overall effectiveness of tamoxifen in the treatment of ER-positive breast cancer.
Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells.[Pubmed:16690721]
J Pharmacol Exp Ther. 2006 Aug;318(2):503-12.
We recently demonstrated that Endoxifen (4-hydroxy-N-desmethyl-tamoxifen), a pharmacogenetically regulated metabolite of tamoxifen, is equipotent to 4-hydroxy-tamoxifen (4-OH-Tam) with respect to estrogen receptor binding and inhibition of 17beta-estradiol (E2)-induced cell proliferation. Endoxifen was also found to be more abundant in human plasma than 4-OH-Tam, and its formation has been shown to be primarily catalyzed by cytochrome P450 2D6 (CYP2D6). Here, we report studies evaluating the effects of Endoxifen, 4-OH-Tam, and E2 on gene expression in MCF-7 cells using Affymetrix U133A GeneChip Arrays (Santa Clara, CA). We detected 4062 genes that were E2-regulated (1924 induced; 2138 suppressed), and the ratio of E2-induced versus E2-suppressed genes was consistent regardless of the cutoff value. In the presence of E2, 2444 and 2390 genes were affected by 4-OH-Tam and Endoxifen, respectively, when no minimal -fold change cutoff was implemented. The majority of genes regulated by the tamoxifen metabolites were also E2-responsive (74.4 and 73.3%, respectively). Endoxifen and 4-OH-Tam had overlapping effects on 1365 E2-sensitive genes, whose -fold effects between these metabolites were highly correlated (R2 = 0.99). A significant correlation was also found between the -fold effects of 249 E2-insensitive genes coregulated by both metabolites (R2 = 0.99). Hierarchical clustering analysis demonstrated similar gene regulation patterns between these metabolites, which were distinct from E2 or vehicle treatment patterns. Using real time-polymerase chain reaction, we validated the gene expression patterns of five genes that were differentially regulated by Endoxifen and 4-OH-Tam. We conclude that Endoxifen and 4-OH-Tam have similar effects on global gene expression patterns in MCF-7 cells and that the majority of the affected genes are estrogen-regulated genes.


