Methyl linoleateCAS# 112-63-0 |
- LINOLELAIDICACIDMETHYLESTER
Catalog No.:BCX0936
CAS No.:2566-97-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 112-63-0 | SDF | Download SDF |
| PubChem ID | 5284421 | Appearance | Powder |
| Formula | C19H34O2 | M.Wt | 294.47 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
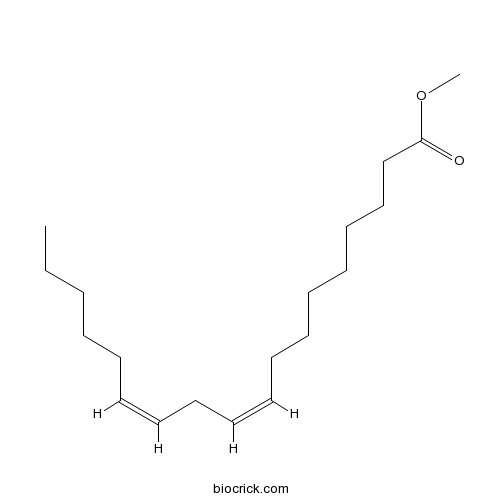
| Chemical Name | methyl (9Z,12Z)-octadeca-9,12-dienoate | ||
| SMILES | CCCCCC=CCC=CCCCCCCCC(=O)OC | ||
| Standard InChIKey | WTTJVINHCBCLGX-NQLNTKRDSA-N | ||
| Standard InChI | InChI=1S/C19H34O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h7-8,10-11H,3-6,9,12-18H2,1-2H3/b8-7-,11-10- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Methyl linoleate has antioxidant activity. |

Methyl linoleate Dilution Calculator

Methyl linoleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3959 mL | 16.9797 mL | 33.9593 mL | 67.9186 mL | 84.8983 mL |
| 5 mM | 0.6792 mL | 3.3959 mL | 6.7919 mL | 13.5837 mL | 16.9797 mL |
| 10 mM | 0.3396 mL | 1.698 mL | 3.3959 mL | 6.7919 mL | 8.4898 mL |
| 50 mM | 0.0679 mL | 0.3396 mL | 0.6792 mL | 1.3584 mL | 1.698 mL |
| 100 mM | 0.034 mL | 0.1698 mL | 0.3396 mL | 0.6792 mL | 0.849 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
- OctMAB
Catalog No.:BCC7893
CAS No.:1120-02-1
- p-Vinylphenyl O-[beta-D-apiofuranosyl-(1-6)]-beta-D-glucopyranoside
Catalog No.:BCN1619
CAS No.:112047-91-3
- Endoxifen
Catalog No.:BCC7761
CAS No.:112093-28-4
- 3-Hydroxy-2-methylpyridine
Catalog No.:BCN8162
CAS No.:1121-25-1
- (S)-(+)-Modafinic acid
Catalog No.:BCC5158
CAS No.:112111-44-1
- (R)-(-)-Modafinic acid
Catalog No.:BCC5157
CAS No.:112111-45-2
- Ikshusterol 3-O-glucoside
Catalog No.:BCN6001
CAS No.:112137-81-2
- DMAP
Catalog No.:BCC2842
CAS No.:1122-58-3
- Pam3CSK4
Catalog No.:BCC6245
CAS No.:112208-00-1
- 14,15-Didehydrovincamenine
Catalog No.:BCN6002
CAS No.:112219-48-4
Diels(-)Alder-Crosslinked Polymers Derived from Jatropha Oil.[Pubmed:30961102]
Polymers (Basel). 2018 Oct 22;10(10). pii: polym10101177.
Methyl oleate, Methyl linoleate, and jatropha oil were fully epoxidized using in situ-generated performic acid. The epoxidized compounds were further reacted with furfurylamine in a solvent-free reaction to obtain furan-functionalized fatty esters which, then, functioned as oligomers for a network preparation. Thermoreversible crosslinking was obtained through a (retro) Diels(-)Alder reaction with bismaleimide, resulting in the formation of a brittle network for furan-functionalized Methyl linoleate and jatropha oil. The furan-functionalized fatty esters were mixed with alternating (1,4)-polyketone reacted with furfurylamine (PK-Furan) for testing the mechanical and self-healing properties with DMTA and DSC, respectively. Full self-healing properties were found, and faster thermoreversibility kinetics were observed, compared to PK-Furan.
Mycorrhiza enhances drought tolerance of citrus by altering root fatty acid compositions and their saturation levels.[Pubmed:30957149]
Tree Physiol. 2019 Apr 5. pii: 5428673.
Arbuscular mycorrhizas (AMs) have the ability to enhance drought tolerance of citrus, while the underlying mechanisms are not clearly elucidated. Considering the strong association of cell membrane fatty acid (FA) unsaturation with plant drought tolerance, the present study hypothesized that arbuscular mycorrhizal fungi (AMF) modulated the composition and unsaturation of FAs to enhance drought tolerance of host plants. A drought-sensitive citrus rootstock, trifoliate orange (Poncirus trifoliata) seedlings, were inoculated with AMF (Funneliformis mosseae) for 3 months, and were subsequently exposed to drought stress (DS) for 8 weeks. Mycorrhizal seedlings exhibited better plant growth performance, higher leaf water potential, and lower root abscisic acid concentrations under both well-watered (WW) and DS, respectively. AMF inoculation considerably increased root methyl oleate (C18:1), Methyl linoleate (C18:2) and methyl linolenate (C18:3 N3) concentrations under both WW and DS and root methyl palmitoleate (C16:1) concentrations under WW, while decreased root methyl stearate (C18:0) levels under both WW and DS. These changes in the composition of FAs of mycorrhized roots resulted in higher unsaturation index of root FAs (UIFA), which later aided in reducing the oxidative damage on account of lower concentration of malondialdehyde and superoxide radicals. The changes of these FAs were a result of AMF-up-regulating root fatty acid desaturase 2 (PtFAD2), fatty acid desaturase 6 (PtFAD6) and Delta9 fatty acid desaturase (PtDelta9) genes under WW and PtFAD2, PtFAD6 and Delta15 fatty acid desaturase (PtDelta15) genes under DS. Our results confirmed that mycorrhization brought significant changes in root FA compositions, in addition to regulation of gene expression responsible for increasing the unsaturation level of FAs, a pre-disposing physiological event for better drought tolerance of citrus.
Antioxidant ability of tyrosol and derivative-compounds in the presence of O2((1)Deltag)-species. Studies of synergistic antioxidant effect with commercial antioxidants.[Pubmed:30797345]
Food Chem. 2019 Jul 1;285:275-281.
The exposure of fatty products to environmental light can trigger lipid oxidation in food through a sensitized-photooxidation process, which involves the participation of the species singlet oxygen (O2((1)Deltag)). Therefore, preservation of food quality represents a subject of great economic interest to the food industry. In this sense, the phenolic compounds are natural antioxidants widely used in food industry. In this contribution we studied the interactions of phenolic derivatives (Phd), tyrosol and tyrosol derived isomers, with O2((1)Deltag) and their possible protective effect against the oxidative degradation of unsaturated fatty acids and amino acids. Besides, a potential synergistic interaction between Phd and antioxidants used in food industry were investigated. Phd substrates showed properties as antioxidant additives due to their high ability deactivating O2((1)Deltag) through a physical process and synergistic effect in the presence of commercial antioxidants. Phd presented an antioxidant protective effect toward O2((1)Deltag)-mediated degradation of Methyl linoleate and tryptophan.
Terretonin O: a new meroterpenoid from Aspergillus terreus.[Pubmed:30602325]
Nat Prod Res. 2019 Jan 3:1-10.
Terretonin O (1), a new meroterpenoid, was isolated individually from both methanolic extracts of thermophilic Aspergillus terreus TM8 and marine Aspergillus terreus LGO13. The recently reported terretonins M (2) and N (3) were further isolated from the fungus LGO13 along with nine known compounds, terrelumamide A (4), terrein (5), methyl-3,4,5-trimethoxyl-2-[2-(nicotinamide)benzamido] benzoate (6), butyrolactones I-III (7-9), aspulvinone O (10), ergosterol, ergost-4-ene-3-one and Methyl linoleate. Structure of terretonin O (1) was established on the bases of HRESIMS, 1D and 2D NMR spectra and comparison with its analogues in literatures. The relative stereochemistry of 1 was assigned on the basis of NOESY spectra and comparison with reported configuration of its congener compounds 2 and 3. The antimicrobial and cytotoxic activities of the fungal extracts and obtained compounds were assayed using a set of microorganisms, and cervix carcinoma cell line (KB-3-1), respectively. Isolation and taxonomical characterization of the producing strains are reported.
Why Not Trans? Inhibited Radical Isomerization Cycles and Coupling Chains of Lipids and Alkenes with Alkane -thiols.[Pubmed:29894181]
J Org Chem. 2018 Aug 17;83(16):9178-9189.
Reversible addition of thiyl radicals to cis fatty acids converts them into trans fatty acids, L Z + S(*) right arrow over left arrow SL(*) right arrow over left arrow L E + S(*), in a cycle that, uninterrupted, would rapidly isomerize lipids exposed to radicals and thiols. One reason this does not happen in foods and organisms is because the cycle is interrupted, by exothermic allylic abstraction, L + S(*) --> L(*) + SH. Autoinhibition limits the cis-trans cycle length to around 400-500 (L E per S(*)) in a MUFA model (methyl oleate) and just approximately 13-15 in a PUFA lipid model (Methyl linoleate). The weak C-H bonds in bisallylic groups in PUFAs thereby act as the first line of defense against thiyl cis-trans cycles in biolipid solutions (+/-O2). With the intriguing exception of vitamin E in MUFA, thiyl-active antioxidants inhibit isomerization in much the same way as they protect against peroxidation. Applied to thiol-ene coupling (TEC), the allylic abstraction, degraded-chain paradigm resolved a raft of hitherto contradictory trends and findings in "click" TEC polymerization and organic synthesis methods.
Characterization of two fungal lipoxygenases expressed in Aspergillus oryzae.[Pubmed:29805113]
J Biosci Bioeng. 2018 Oct;126(4):436-444.
Two fungal lipoxygenase genes were cloned from a rice pathogen, Magnaporthe salvinii, and the take-all fungus, Gaeumannomyces graminis var. tritici, and successfully expressed in Aspergillus oryzae in secreted form. The lipoxygenases expressed, termed MLOX and GLOX, were purified and characterized to evaluate suitability for industrial applications. Both enzymes were active broadly at pH 4-11 and had optimum temperatures around 60 degrees C, but they were largely different in substrate specificity. Where MLOX was active broadly on arachidonic acid, EPA and DHA, and even on derivatives of fatty acids, such as Methyl linoleate or linoleoyl alcohol, GLOX was more specific to linoleic acid and linolenic acid. The most remarkable difference between the two fungal LOXs was the positional and stereo-specificity of oxygenation reactions on polyunsaturated fatty acids. When using linoleic acid as the substrate, the product of MLOX was 9S-hydroperoxy-(E,Z)-octadecadienoic acid (9S(E,Z)-HPODE), on the other hand, the product of GLOX was 13R(E,Z)-HPODE. The enzymes were evaluated for a couple of potential applications and found to be effective on bleaching colored compounds such as carotenoids.
Antioxidant activity and calcium binding of isomeric hydroxybenzoates.[Pubmed:29567228]
J Food Drug Anal. 2018 Apr;26(2):591-598.
The association constant for calcium binding to hydroxybenzoates in aqueous 0.16 M NaCl at 25 degrees C was found electrochemically to have the value Kass = 280 mol L(-1) with DeltaH(o) = -51 kJ mol(-1), DeltaS(o) = -122 J mol(-1) K(-1) for the 2-isomer (salicylate), Kass = 7 mol L(-1) with DeltaH(o) = -39 kJ mol(-1), DeltaS(o) = -116 J mol(-1) K(-1) for the 3-isomer, and Kass = 8 mol L(-1) with DeltaH(o) = -51 kJ mol(-1), DeltaS(o) = -155 J mol(-1) K(-1) for the 4-isomer. The 3- and 4-isomers were found more efficient as antioxidants than the 2-isomer in decreasing oxygen consumption rate in a peroxidating Methyl linoleate emulsion and less sensitive to presence of calcium. All isomers were found prooxidative for iron-catalyzed initiation of oxidation due to enhanced radical formation as shown by electron spin resonance spectroscopy. Calcium salicylate was found to have low solubility with a solubility product Ksp = 4.49.10(-6) based on activity with DeltaH(o) = 67 kJ mol(-1), DeltaS(o) = 123 J mol(-1) K(-1) for dissolution in water, when corrected for the strong complex formation. Calcium in food and beverages may thus lower antioxidant activity of plant phenols through complexation or by precipitation.
Statistical optimization for lipase production from solid waste of vegetable oil industry.[Pubmed:29424632]
Prep Biochem Biotechnol. 2018 Apr 21;48(4):321-326.
The production of biofuel using thermostable bacterial lipase from hot spring bacteria out of low-cost agricultural residue olive oil cake is reported in the present paper. Using a lipase enzyme from Bacillus licheniformis, a 66.5% yield of methyl esters was obtained. Optimum parameters were determined, with maximum production of lipase at a pH of 8.2, temperature 50.8 degrees C, moisture content of 55.7%, and biosurfactant content of 1.693 mg. The contour plots and 3D surface responses depict the significant interaction of pH and moisture content with biosurfactant during lipase production. Chromatographic analysis of the lipase transesterification product was methyl esters, from kitchen waste oil under optimized conditions, generated methyl palmitate, methyl stearate, methyl oleate, and Methyl linoleate.
Sageretia thea fruit extracts rich in methyl linoleate and methyl linolenate downregulate melanogenesis via the Akt/GSK3beta signaling pathway.[Pubmed:29399291]
Nutr Res Pract. 2018 Feb;12(1):3-12.
BACKGROUND/OBJECTIVES: Sageretia thea is traditionally used as a medicinal herb to treat various diseases, including skin disorders, in China and Korea. This study evaluated the inhibitory effect of Sageretia thea fruit on melanogenesis and its underlying mechanisms in B16F10 mouse melanoma cells. The active chemical compounds in anti-melanogenesis were determined in Sageretia thea. MATERIALS/METHODS: Solvent fractions from the crude extract were investigated for anti-melanogenic activities. These activities and the mechanism of anti-melanogenesis in B16F10 cells were examined by determining melanin content and tyrosinase activity, and by performing western blotting. RESULTS: The n-hexane fraction of Sageretia thea fruit (HFSF) exhibited significant anti-melanogenic activity among the various solvent fractions without reducing viability of B16F10 cells. The HFSF suppressed the expression of tyrosinase and tyrosinase-related protein 1 (TRP1). The reduction of microphthalmia-associated transcription factor (MITF) expression by the HFSF was mediated by the Akt/glycogen synthase kinase 3 beta (GSK3beta) signaling pathway, which promotes the reduction of beta-catenin. Treatment with the GSK3beta inhibitor 6-bromoindirubin-3'-oxime (BIO) restored HFSF-induced inhibition of MITF expression. The HFSF bioactive constituents responsible for anti-melanogenic activity were identified by bioassay-guided fractionation and gas chromatography-mass spectrometry analysis as Methyl linoleate and methyl linolenate. CONCLUSIONS: These results indicate that HFSF and its constituents, Methyl linoleate and methyl linolenate, could be used as whitening agents in cosmetics and have potential for treating hyperpigmentation disorders in the clinic.
Herbicidal Activities of Some Allelochemicals and Their Synergistic Behaviors toward Amaranthus tricolor L.[Pubmed:29077029]
Molecules. 2017 Oct 27;22(11). pii: molecules22111841.
Seven allelochemicals, namely R-(+)-limonene (A), vanillin (B), xanthoxyline (C), vanillic acid (D), linoleic acid (E), Methyl linoleate (F), and (+/-)-odorine (G), were investigated for their herbicidal activities on Chinese amaranth (Amaranthus tricolor L.). At 400 muM, xanthoxyline (C) showed the greatest inhibitory activity on seed germination and seedling growth of the tested plant. Both vanillic acid (D) and (+/-)-odorine (G) inhibited shoot growth, however, apart from xanthoxyline (C), only vanillic acid (D) could inhibit root growth. Interestingly, R-(+)-limonene (A) lightly promoted root length. Other substances had no allelopathic effect on seed germination and seedling growth of the tested plant. To better understand and optimize the inhibitory effects of these natural herbicides, 21 samples of binary mixtures of these seven compounds were tested at 400 muM using 0.25% (v/v) Tween((R)) 80 as a control treatment. The results showed that binary mixtures of R-(+)-limonene:xanthoxyline (A:C), vanillin:xanthoxyline (B:C), and xanthoxyline:linoleic acid (C:E) exhibited strong allelopathic activities on germination and seedling growth of the tested plant, and the level of inhibition was close to the effect of xanthoxyline (C) at 400 microM and was better than the effect of xanthoxyline (C) at 200 microM. The inhibition was hypothesized to be from a synergistic interaction of each pair of alleochemicals. Mole ratios of each pair of allelochemicals ((A:C), (B:C), and (C:E)) were then evaluated, and the best ratios of the binary mixtures A:C, B:C and C:E were found to be 2:8, 2:8, and 4:6 respectively. These binary mixtures significantly inhibited germination and shoot and root growth of Chinese amaranth at low concentrations. The results reported here highlight a synergistic behavior of some allelochemicals which could be applied in the development of potential herbicides.
Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils.[Pubmed:28876392]
An Acad Bras Cienc. 2017 Jul-Sep;89(3):1671-1681.
Fatty acid methyl esters (FAMEs) were obtained from vegetable oils of soybean, corn and sunflower. The current study was focused on evaluating the antifungal activity of FAMEs mainly against Paracoccidioides spp., as well as testing the interaction of these compounds with commercial antifungal drugs and also their antioxidant potential. FAMEs presented small IC50 values (1.86-9.42 mug/mL). All three FAMEs tested showed antifungal activity against isolates of Paracoccidioides spp. with MIC values ranging from 15.6-500 microg/mL. Sunflower FAMEs exhibited antifungal activity that extended also to other genera, with an MIC of 15.6 mug/mL against Candida glabrata and C. krusei and 31.2 mug/mL against C. parapsilosis. FAMEs exhibited a synergetic effect with itraconazole. The antifungal activity of the FAMEs against isolates of Paracoccidioides spp. is likely due to the presence of Methyl linoleate, the major compound present in all three FAMEs. The results obtained indicate the potential of FAMEs as sources for antifungal and antioxidant activity.
A flow-based procedure exploiting the lab-in-syringe approach for the determination of ester content in biodiesel and diesel/biodiesel blends.[Pubmed:28738622]
Talanta. 2017 Nov 1;174:556-561.
The ester content is an important parameter to be monitored in biodiesel for evaluation of the transesterification reaction yield and for assessing the purity of the final product. This is also a relevant quality parameter in diesel/biodiesel blends to avoid frauds, because legislation establishes a minimum amount of biodiesel to be added to diesel. The official method EN14103 requires the addition of an alternative internal standard (methyl nonadecanoate) for analysis of biodiesel from bovine tallow because the methyl heptadecanoate is found in high amounts in this product. In this work, it is proposed a fast, simple, practical, and environmental friendly flow-based spectrophotometric procedure, which exploits the formation of the violet complex between Fe(III) and the hydroxamate generated by the reactions of the alkyl esters with hydroxylamine. All involved steps are carried out inside the syringe pump of a sequential injection analyzer (lab-in-syringe approach). A single phase is attained by using ethanol as mediator solvent between the organic sample and aqueous soluble reagents. Linear responses for biodiesel samples and diesel/biodiesel blends were obtained from 4-99%(v/v) to 2.0-40%(v/v) methyl esters, described by the equations: A = 0.342 + 0.00305C (r = 0.997) and A = 0.174 + 0.00503C (r = 0.999), respectively. The analytical curve can be obtained by in-line dilution of a Methyl linoleate stock solution. For biodiesel samples, the coefficient of variation (n = 10), limit of detection (99.7% confidence level), and sampling rate were estimated at 0.8%, 0.36%(v/v), and 15h(-1), respectively, whereas the corresponding values for the blend samples were 0.20%, 0.03%(v/v), and 12h(-1), respectively. The procedure consumes only 860mug of hydroxylamine, 366mug of Fe2(SO4)3.H2O, and 2.0mL ethanol and generates ca. 3.0mL of residue per determination. The results agreed with those obtained by official methods EN14103/2011 e EN14078, at the 95% confidence level.
Metabolite analysis of endophytic fungi from cultivars of Zingiber officinale Rosc. identifies myriad of bioactive compounds including tyrosol.[Pubmed:28597159]
3 Biotech. 2017 Jun;7(2):146.
Endophytic fungi associated with rhizomes of four cultivars of Zingiber officinale were identified by molecular and morphological methods and evaluated for their activity against soft rot pathogen Pythium myriotylum and clinical pathogens. The volatile bioactive metabolites produced by these isolates were identified by GC-MS analysis of the fungal crude extracts. Understanding of the metabolites produced by endophytes is also important in the context of raw consumption of ginger as medicine and spice. A total of fifteen isolates were identified from the four varieties studied. The various genera identified were Acremonium sp., Gliocladiopsis sp., Fusarium sp., Colletotrichum sp., Aspergillus sp., Phlebia sp., Earliella sp., and Pseudolagarobasidium sp. The endophytic community was unique to each variety, which could be due to the varying host genotype. Fungi from phylum Basidiomycota were identified for the first time from ginger. Seven isolates showed activity against Pythium, while only two showed antibacterial activity. The bioactive metabolites identified in the fungal crude extracts include tyrosol, benzene acetic acid, ergone, dehydromevalonic lactone, N-aminopyrrolidine, and many bioactive fatty acids and their derivatives which included linoleic acid, oleic acid, myristic acid, n-hexadecanoic acid, palmitic acid methyl ester, and Methyl linoleate. The presence of these varying bioactive endophytic fungi may be one of the reasons for the differences in the performance of the different ginger varieties.


