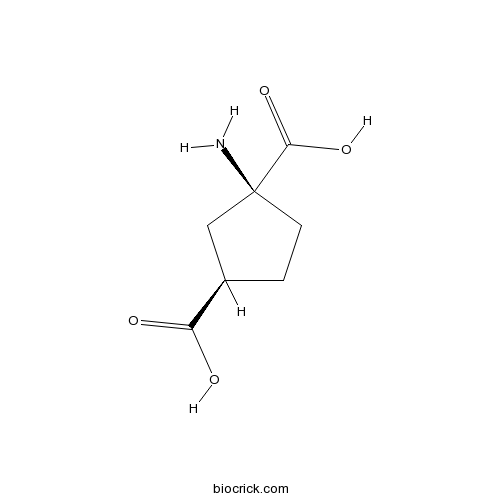
(1S,3R)-ACPDGroup II/group I mGlu agonist CAS# 111900-32-4 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111900-32-4 | SDF | Download SDF |
| PubChem ID | 104766 | Appearance | Powder |
| Formula | C7H11NO4 | M.Wt | 173.17 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 30 mM in water | ||
| Chemical Name | (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid | ||
| SMILES | C1CC(CC1C(=O)O)(C(=O)O)N | ||
| Standard InChIKey | YFYNOWXBIBKGHB-FBCQKBJTSA-N | ||
| Standard InChI | InChI=1S/C7H11NO4/c8-7(6(11)12)2-1-4(3-7)5(9)10/h4H,1-3,8H2,(H,9,10)(H,11,12)/t4-,7+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Active isomer of (±)-trans-ACPD. Agonist at both group I and II mGlu receptors (EC50 values are 5, 15, 42 and 60 μM at mGluR2, mGluR5, mGluR1 and mGluR6 respectively). NPEC-caged-(1S,3R)-ACPD also available . (±)-trans-ACPD and cis-ACPD also available. |

(1S,3R)-ACPD Dilution Calculator

(1S,3R)-ACPD Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7747 mL | 28.8734 mL | 57.7467 mL | 115.4934 mL | 144.3668 mL |
| 5 mM | 1.1549 mL | 5.7747 mL | 11.5493 mL | 23.0987 mL | 28.8734 mL |
| 10 mM | 0.5775 mL | 2.8873 mL | 5.7747 mL | 11.5493 mL | 14.4367 mL |
| 50 mM | 0.1155 mL | 0.5775 mL | 1.1549 mL | 2.3099 mL | 2.8873 mL |
| 100 mM | 0.0577 mL | 0.2887 mL | 0.5775 mL | 1.1549 mL | 1.4437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
- Acetic acid octyl ester
Catalog No.:BCN8303
CAS No.:112-14-1
- Methyl hexadecanoate
Catalog No.:BCN8290
CAS No.:112-39-0
- Methyl Stearate
Catalog No.:BCN8309
CAS No.:112-61-8
- Methyl Oleate
Catalog No.:BCN8306
CAS No.:112-62-9
- Methyl linoleate
Catalog No.:BCN8137
CAS No.:112-63-0
- Oleic acid
Catalog No.:BCN7159
CAS No.:112-80-1
- Docosanoic acid
Catalog No.:BCC8952
CAS No.:112-85-6
(1S,3R)-ACPD, a metabotropic glutamate receptor agonist, enhances damage after global ischaemia.[Pubmed:9988123]
Eur J Pharmacol. 1999 Jan 15;365(1):55-8.
There are opposing results in the literature concerning the influence of (1S,3R)-ACPD [(1S,3R)-1-aminocyclopentane-1,3-dicarboxylate: group I/II metabotropic glutamate receptor agonist) on neurodegeneration, showing both enhancement and reduction of damage. We examined the effects of (1S,3R)-ACPD, given in various application schedules, on global ischaemia in gerbils. The most pronounced effect was a significant increase of hippocampal damage when the drug was applied at 20 mg/kg i.p. pre ischaemia. All other experiments with lower concentrations (0.02-2 mg/kg), other time schedules (post-ischaemic application) or co-application of a selective group I metabotropic glutamate receptor antagonist (4-CPG: (S)-4-carboxyphenylglycine), had no influence on neuronal density.
Metabotropic glutamate receptor agonist (1S,3R-ACPD) increased frontal cortex dopamine release in aged but not in young rats.[Pubmed:9832384]
Eur J Pharmacol. 1998 Oct 23;359(2-3):139-42.
The effects of the metabotropic glutamate (mGlu) receptor agonist (1S,3R)-1-Amino cyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) infusion on frontal cortex dopamine extracellular levels were studied by microdialysis in young (3 months) and aged (24 months) rats. Basal dopamine levels were significantly higher in young than in aged rats. (1S,3R)-ACPD (1 mM) significantly increased dopamine efflux in aged rats, an effect which was antagonized by the mGlu receptor antagonist, (S)-alpha-methyl-4-carboxypheniylglycine (MCPG) (2 mM). On the contrary, (IS,3R)-ACPD up to the concentration of 2 mM failed to influence dopamine extracellular levels in young rats. These results suggest that the agonist of mGlu receptor group I and/or II can improve dopamine release under conditions of deficiency of extracellular dopamine concentration as observed in aging.
Group II metabotropic glutamate receptors are a common target of N-anisoyl-GABA and 1S,3R-ACPD in enhancing ACh release in the prefrontal cortex of freely moving SHRSP.[Pubmed:10699452]
Neuropharmacology. 2000 Mar 3;39(5):866-72.
Aniracetam is a therapeutically useful cognition enhancer for treating various neuropsychiatric symptoms occurring after cerebral infarction. We recently reported that local perfusion of its major metabolites N-anisoyl-GABA and p-anisic acid, but not aniracetam itself, enhanced acetylcholine (ACh) release with a delayed onset in cerebral regions of stroke-prone spontaneously hypertensive rats (SHRSP). In this study, we examined the possible involvement of metabotropic and ionotropic glutamate (mGlu and AMPA) receptors in the N-anisoyl-GABA-induced ACh release using brain in vivo microdialysis. Basal ACh release in SHRSP was commonly lower in the nucleus reticularis thalami, dorsal hippocampus and prefrontal cortex than that in age-matched Wistar Kyoto rats. The delayed ACh release in the prefrontal cortex of SHRSP was completely blocked by MCPG, a group I and II mGlu receptor antagonist, and MCCG, a group II-selective mGlu receptor antagonist. In contrast, it was largely unaffected by AIDA, a group I-selective mGlu receptor antagonist, or by YM90K, an AMPA receptor antagonist. 1S,3R-ACPD, a preferential group II mGlu receptor agonist, enhanced ACh release with a similar latency and the effect was antagonized by MCCG, whereas AMPA induced a prompt ACh release. These results indicate that N-anisoyl-GABA and 1S,3R-ACPD share a common mechanism mediated by group II mGlu receptors in enhancing ACh release. The findings suggest a possible mechanism for aniracetam's clinical efficacy in stroke patients with cholinergic deficits.
The metabotropic glutamate receptor agonist 1S,3R-ACPD stimulates and modulates NMDA receptor mediated excitotoxicity in organotypic hippocampal slice cultures.[Pubmed:11292452]
Brain Res. 2001 Apr 13;898(1):91-104.
The potential toxic effects of the metabotropic glutamate receptor agonist (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) and its interactions with the N-methyl-D-aspartate (NMDA) receptor were studied in hippocampal brain slice cultures, using densitometric measurements of the cellular uptake of propidium iodide (PI) to quantify neuronal degeneration. Cultures exposed to ACPD, showed a concentration (2-5 mM) and time (1-4 days) dependent increase in PI uptake in CA1, CA3 and dentate subfields after 24 h and 48 h of exposure, with CA1 pyramidal cells being most sensitive. The neurodegeneration induced by 2 mM ACPD was completely abolished by addition of 10 microM of the NMDA receptor antagonist (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK-801), while 20 microM of the 2-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainic acid receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX) had no effect. Co-exposing cultures to a subtoxic dose of 300 microM ACPD together with 10 microM NMDA, which at this dose is known to induce a fairly selective degeneration of CA1 pyramidal cells, significantly increased the PI uptake in both CA1 and CA3, compared to cultures exposed to 10 microM NMDA only. Adding the 300 microM ACPD as pretreatment for 30 min followed by a 30 min wash in normal medium before the ACPD/NMDA co-exposure, eliminated the potentiation of NMDA toxicity. The potentiation was also blocked by addition of 10 or 100 microM 2-methyl-6-(phenylethynyl)pyridine (MPEP) (mGluR5 antagonist) during the co-exposure, while a corresponding addition of 10 or 100 microM 7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) (mGluR1 antagonist) had no effect. We conclude that, stimulation of metabotropic glutamate receptors with ACPD at concentrations of 2 mM or higher induces a distinct subfield-related and time and concentration dependent pattern of hippocampal degeneration, and that ACPD at subtoxic concentrations modulates NMDA-induced excitotoxicity through the mGluR5 receptor in a time dependent way.
Synthesis and pharmacology of 3-hydroxy-delta2-isoxazoline-cyclopentane analogues of glutamic acid.[Pubmed:12484537]
Farmaco. 2002 Nov;57(11):889-95.
The synthesis and pharmacology of two potential glutamic acid receptor ligands are described. Preparation of the bicyclic 3-hydroxy-delta2-isoxazoline-cyclopentane derivatives (+/-)-7 and (+/-)-8 was accomplished via 1,3-dipolar cycloaddition of bromonitrile oxide to suitably protected 1-amino-cyclopent-3-enecarboxylic acids. Their structure was established using a combination of 1H NMR spectroscopy and molecular mechanics calculations carried out on the intermediate cycloadducts (+/-)-11 and (+/-)-12. Amino acid derivatives (+/-)-7 and (+/-)-8 were assayed at ionotropic and metabotropic glutamic acid receptor subtypes and their activity compared with that of trans-ACPD and cis-ACPD. The results show that the replacement of the omega-carboxylic group of the model compounds with the 3-hydroxy-delta2-isoxazoline moiety abolishes or reduces drastically the activity at the metabotropic glutamate receptors. Conversely, on passing from cis-ACPD to derivative (+/-)-8, the agonist activity at NMDA receptors is almost unaffected.
Differences in agonist and antagonist activities for two indices of metabotropic glutamate receptor-stimulated phosphoinositide turnover.[Pubmed:8732284]
Br J Pharmacol. 1996 Apr;117(8):1735-43.
1. The abilities of the four diastereoisomers of 1-aminocyclopentane-1,3-dicarboxylic acid (ACPD) to stimulate, and the metabotropic glutamate receptor (mGluR) antagonist (+/-)-alpha-methylcarboxyphenylglycine (MCPG) to inhibit, phosphoinositide turnover in neonatal rat cerebral cortex have been studied. Two indices of phosphoinositide cycle activity were assessed; inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) mass accumulation, and total inositol phosphate [3H]-InsPx accumulation (in the presence of Li+) in myo-[3H]-inositol prelabelled slices. 2. The diastereoisomers of ACPD stimulated each response with a rank order of potency of 1S, 3R > 1R, 3R > 1S, 3S >> 1R, 3S. The response to 1R, 3R-ACPD was largely prevented by pre-addition of the NMDA-receptor antagonist, MK-801, or omission of extracellular Ca2+, suggesting that this isomer acts indirectly on phosphoinositide responses through activation of NMDA-type ionotropic glutamate receptors. In contrast, the responses to 1S, 3R- and 1S, 3S-ACPD were unaffected by prior addition of MK-801, but were blocked by MCPG. 3. The concentration of 1S, 3R-ACPD required to half-maximally stimulate the Ins(1,4,5)P3 response (-log EC50 (M), -4.09 +/- 0.10) was significantly higher than that required to exert a similar effect on [3H]-InsPx accumulation (-log EC50 (M), -4.87 +/- 0.07; P < 0.01; n = 4). A similar marked 8-9 fold discrepancy between these two values was observed for the 1S, 3S isomer, which elicited similar maximal responses to those caused by 1S, 3R-ACPD. 4. Significant differences were also observed with respect to the ability of (+/-)-MCPG (1 mM) to cause a rightward shift in the concentration-response relationships for 1S, 3R-ACPD-stimulated Ins(1,4,5)P3 (5.59 +/- 0.24 fold shift) and [3H]-InsPx (3.04 +/- 0.34 fold shift; P < 0.01; n = 4) responses, giving rise to Kd values of 218 and 490 microM for (+/-)-MCPG antagonism of the respective responses. 5. The potency difference between the 1S, 3R-ACPD-stimulated Ins(1,4,5)P3 and [3H]-InsPx responses was reduced when experiments were performed in nominally calcium-free medium ([Ca2+]e = 2 - 5 microM) and EC50 values were almost identical when extracellular calcium was reduced further by EGTA addition ([Ca2+]e < or = 100 nM). Similarly, the Kd value for (+/-)-MCPG antagonism of the 1S, 3R-ACPD-stimulated [3H]-InsPx response decreased under [Ca2+]e-free conditions, approaching those obtained for the 1S, 3R-ACPD-stimulated Ins(1,4,5)P3 response in the presence of normal [Ca2+]e. 6. These data suggest that estimates of the activities of mGluR agonists and antagonists, derived by measuring phosphoinositide turnover, can differ significantly depending on whether Ins(1,4,5)P3 mass or [3H]-InsPx responses are measured. In particular, the possibility that the mGluR-mediated [3H]-InsPx response may not simply reflect direct receptor/G protein/phosphoinositidase C (PIC) activation, but may also be the consequence of stimulation of a facilitatory Ca2+-influx pathway is discussed.


