10-O-Methylprotosappanin BCAS# 111830-77-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111830-77-4 | SDF | Download SDF |
| PubChem ID | 124355840 | Appearance | Powder |
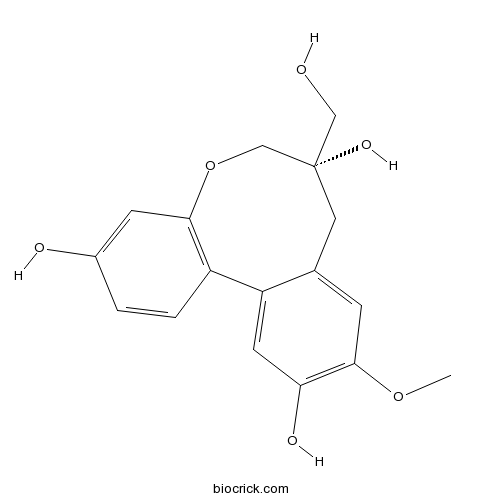
| Formula | C17H18O6 | M.Wt | 318.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | COC1=C(C=C2C(=C1)CC(COC3=C2C=CC(=C3)O)(CO)O)O | ||
| Standard InChIKey | MPBIWBGTEYMVRN-KRWDZBQOSA-N | ||
| Standard InChI | InChI=1S/C17H18O6/c1-22-16-4-10-7-17(21,8-18)9-23-15-5-11(19)2-3-12(15)13(10)6-14(16)20/h2-6,18-21H,7-9H2,1H3/t17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| Structure Identification | Chem. Pharm. Bull.,1987,35(9):3615-9.Homoisoflavonoids and Related Compounds. V.1) A Novel Dibenzoxocin Derivative from Caesalpinia sappan L[Reference: WebLink]We have been further studying the isolation, absolute stereochemistries and biosyntheses of the phenolic constituents of C. sappan and C. japonica.
|

10-O-Methylprotosappanin B Dilution Calculator

10-O-Methylprotosappanin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1417 mL | 15.7085 mL | 31.4169 mL | 62.8338 mL | 78.5423 mL |
| 5 mM | 0.6283 mL | 3.1417 mL | 6.2834 mL | 12.5668 mL | 15.7085 mL |
| 10 mM | 0.3142 mL | 1.5708 mL | 3.1417 mL | 6.2834 mL | 7.8542 mL |
| 50 mM | 0.0628 mL | 0.3142 mL | 0.6283 mL | 1.2567 mL | 1.5708 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3142 mL | 0.6283 mL | 0.7854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- Remacemide hydrochloride
Catalog No.:BCC7129
CAS No.:111686-79-4
- Elastase Inhibitor
Catalog No.:BCC1225
CAS No.:111682-13-4
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- Nyasicol
Catalog No.:BCN5999
CAS No.:111518-95-7
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
Homoisoflavonoids and Related Compounds. V.1) A Novel Dibenzoxocin Derivative from Caesalpinia sappan L
Chem. Pharm. Bull.,1987,35(9):3615-9.
We have been further studying the isolation, absolute stereochemistries and biosyntheses of the phenolic constituents of C. sappan and C. japonica. In the present paper,we report the isolation and structural elucidation of a novel dibenzoxocin derivative,10-O-Methylprotosappanin B(1), from Sappan Lignum.10-O-Methylprotosappanin B (1) was obtained by repeated silica gel and Sephadex LH-20 column chromatography, and preparative thin-layer chromatography (PTLC) of the remaining fractions after separation of the previously reported compounds.4) The isolation of the intact compound was so difficult that only a limited amount could be obtained. However, a satisfactory amount was obtained in the form of an isopropylidene derivative (6) from the methanolic extract separated after treatment with acetone in the presence of an acid catalyst, as described in the previous paper.4c) The remaining fractions after isolation of the isopropylidene derivatives of 3,4-dihydroxy homoisoflavans4c) were subjected to column chromatography on silica gel and Sephadex LH-20 to yield 6, together with the isopropylidene derivative of protosappanin B (7). On acid hydrolysis with 60% AcOH, compound 6 was readily transformed into 1


