UCPH 101Selective non-substrate EAAT1 inhibitor CAS# 1118460-77-7 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1118460-77-7 | SDF | Download SDF |
| PubChem ID | 25223366 | Appearance | Powder |
| Formula | C27H22N2O3 | M.Wt | 422.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (236.70 mM; Need ultrasonic) | ||
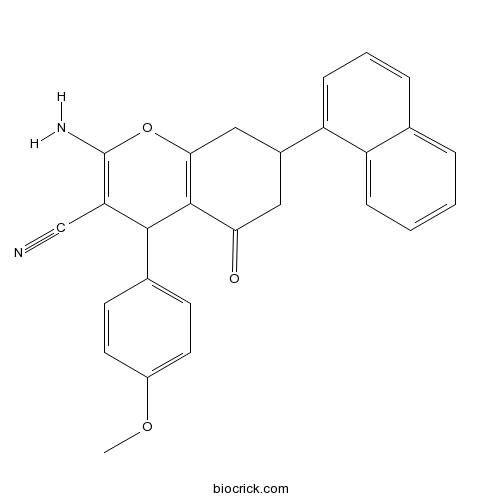
| Chemical Name | 2-amino-4-(4-methoxyphenyl)-7-naphthalen-1-yl-5-oxo-4,6,7,8-tetrahydrochromene-3-carbonitrile | ||
| SMILES | COC1=CC=C(C=C1)C2C(=C(OC3=C2C(=O)CC(C3)C4=CC=CC5=CC=CC=C54)N)C#N | ||
| Standard InChIKey | YBMGNDPBARCLFT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H22N2O3/c1-31-19-11-9-17(10-12-19)25-22(15-28)27(29)32-24-14-18(13-23(30)26(24)25)21-8-4-6-16-5-2-3-7-20(16)21/h2-12,18,25H,13-14,29H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective non-substrate inhibitor of EAAT1 (IC50 values are 660, >300000 and >300000 nM for EAAT1, EAAT2 and EAAT3 respectively). Also demonstrates no significant inhibition at EAAT4 or EAAT5 in a patch-clamp electrophysiology assay (at final concentration up to 10 μM). |

UCPH 101 Dilution Calculator

UCPH 101 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.367 mL | 11.8349 mL | 23.6698 mL | 47.3395 mL | 59.1744 mL |
| 5 mM | 0.4734 mL | 2.367 mL | 4.734 mL | 9.4679 mL | 11.8349 mL |
| 10 mM | 0.2367 mL | 1.1835 mL | 2.367 mL | 4.734 mL | 5.9174 mL |
| 50 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9468 mL | 1.1835 mL |
| 100 mM | 0.0237 mL | 0.1183 mL | 0.2367 mL | 0.4734 mL | 0.5917 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- Remacemide hydrochloride
Catalog No.:BCC7129
CAS No.:111686-79-4
- Elastase Inhibitor
Catalog No.:BCC1225
CAS No.:111682-13-4
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
Probing for improved potency and in vivo bioavailability of excitatory amino acid transporter subtype 1 inhibitors UCPH-101 and UCPH-102: design, synthesis and pharmacological evaluation of substituted 7-biphenyl analogs.[Pubmed:24682739]
Neurochem Res. 2014 Oct;39(10):1964-79.
Uptake of the major excitatory neurotransmitter in the CNS, (S)-glutamate, is mediated by a family of excitatory amino acid transporters (EAAT). Previously we have explored the structure-activity relationship (SAR) of a series of EAAT1 selective inhibitors, leading to the development of the potent inhibitors UCPH-101 and UCPH-102. In the present study, we set out to improve the solubility properties of these EAAT1 inhibitors with the objective to develop analogs more suited as pharmacological tools for in vivo studies of EAAT1 in terms of their bioavailability. A total of 23 novel UCPH-101/102 analogs were designed, synthesized and characterized pharmacologically at EAAT1-3 in a [(3)H]-D-aspartate uptake assay. Most notably, the potent EAAT1 inhibition displayed of UCPH-101 and UCPH-102 was retained in analog 1d in which the napht-1-yl group in the 7-position of UCPH-102 has been replaced by an o-biphenyl moiety. In contrast, EAAT1 activity was dramatically compromised in analogs 1e and 1f comprising m- and p-biphenyl groups as 7-substituents, respectively. Analog 1d displayed low bioavailability after oral administration in rats, and this problem was addressed by the synthesis of a series of analogs with different chloro, fluoro, methoxy, triflouromethyl and carboxy substitution patterns at the o-biphenyl group of 1d (1h-1s) and m- and p-pyridine analogs of 1d (1t and 1v). Unfortunately, all of the modifications resulted in substantial decreased EAAT1 inhibitory activity, which supports the notion of a very lipophilic binding pocket in EAAT1 for the aromatic 7-substituent in these ligands. In conclusion, while we have not succeeded in developing UCPH-101/102 analogs possessing improved bioavailability properties, this study does offer interesting SAR information about this inhibitor class, and analog 1d seems to be an interesting lead for future SAR studies with focus on the development of more potent EAAT1 inhibitors.
Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain.[Pubmed:23325245]
J Neurosci. 2013 Jan 16;33(3):1068-87.
In the present study, the mechanism of action and molecular basis for the activity of the first class of selective inhibitors of the human excitatory amino acid transporter subtype 1 (EAAT1) and its rodent ortholog GLAST are elucidated. The previously reported specificity of UCPH-101 and UCPH-102 for EAAT1 over EAAT2 and EAAT3 is demonstrated to extend to the EAAT4 and EAAT5 subtypes as well. Interestingly, brief exposure to UCPH-101 induces a long-lasting inactive state of EAAT1, whereas the inhibition exerted by closely related analogs is substantially more reversible in nature. In agreement with this, the kinetic properties of UCPH-101 unblocking of the transporter are considerably slower than those of UCPH-102. UCPH-101 exhibits noncompetitive inhibition of EAAT1, and its binding site in GLAST has been delineated in an elaborate mutagenesis study. Substitutions of several residues in TM3, TM4c, and TM7a of GLAST have detrimental effects on the inhibitory potency and/or efficacy of UCPH-101 while not affecting the pharmacological properties of (S)-glutamate or the competitive EAAT inhibitor TBOA significantly. Hence, UCPH-101 is proposed to target a predominantly hydrophobic crevice in the "trimerization domain" of the GLAST monomer, and the inhibitor is demonstrated to inhibit the uptake through the monomer that it binds to exclusively and not to affect substrate translocation through the other monomers in the GLAST trimer. The allosteric mode of UCPH-101 inhibition underlines the functional importance of the trimerization domain of the EAAT and demonstrates the feasibility of modulating transporter function through ligand binding to regions distant from its "transport domain."
New Insight into the Structure-Activity Relationships of the Selective Excitatory Amino Acid Transporter Subtype 1 (EAAT1) Inhibitors UCPH-101 and UCPH-102.[Pubmed:26757239]
ChemMedChem. 2016 Feb 17;11(4):382-402.
In the present study, we made further investigations on the structure-activity requirements of the selective excitatory amino acid transporter 1 (EAAT1) inhibitor, 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101), by exploring 15 different substituents (R(1) ) at the 7-position in combination with eight different substituents (R(2) ) at the 4-position. Among the 63 new analogues synthesized, we identified a number of compounds that unexpectedly displayed inhibitory activities at EAAT1 in light of understanding the structure-activity relationship (SAR) of this inhibitor class extracted from previous studies. Moreover, the nature of the R(1) and R(2) substituents were observed to contribute to the functional properties of the various analogues in additive and non-additive ways. Finally, separation of the four stereoisomers of analogue 14 g (2-amino-4-([1,1'-biphenyl]-4-yl)-3-cyano-7-isopropyl-5-oxo-5,6,7,8-tetrahydro-4H -chromene) was carried out, and in agreement with a study of a related scaffold, the R configuration at C4 was found to be mandatory for inhibitory activity, while both the C7 diastereomers were found to be active as EAAT1 inhibitors. A study of the stereochemical stability of the four pure stereoisomers 14 g-A-D showed that epimerization takes places at C7 via a ring-opening, C-C bond rotation, ring-closing mechanism.
Design, synthesis and pharmacological characterization of coumarin-based fluorescent analogs of excitatory amino acid transporter subtype 1 selective inhibitors, UCPH-101 and UCPH-102.[Pubmed:23072958]
Bioorg Med Chem. 2012 Dec 1;20(23):6831-9.
The excitatory amino acid transporters (EAATs) play a pivotal role in regulating the synaptic concentration of glutamate in the mammalian central nervous system. To date, five different subtypes have been identified, named EAAT15 in humans (and GLAST, GLT-1, EAAC1, EAAT4, and EAAT5, respectively, in rodents). Recently, we have published and presented a structure-activity relationship (SAR) study of a novel class of selective inhibitors of EAAT1 (and GLAST), with the analogs UCPH-101 (IC(50)=0.66muM) and UCPH-102 (IC(50)=0.43muM) being the most potent inhibitors in the series. In this paper, we present the design, synthesis and pharmacological evaluation of six coumarin-based fluorescent analogs of UCPH-101/102 as subtype-selective inhibitors at EAAT1. Analogs 1114 failed to inhibit EAAT1 function (IC(50) values >300muM), whereas analogs 15 and UCPH-102F inhibited EAAT1 with IC(50) values in the medium micromolar range (17muM and 14muM, respectively). Under physiological pH no fluorescence was observed for analog 15, while a bright blue fluorescence emission was observed for analog UCPH-102F. Regrettably, under confocal laser scanning microscopy selective visualization of expression of EAAT1 over EAAT3 was not possible due to nonspecific binding of UCPH-102F.
Structure-activity relationship study of first selective inhibitor of excitatory amino acid transporter subtype 1: 2-Amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101).[Pubmed:20857912]
J Med Chem. 2010 Oct 14;53(19):7180-91.
The excitatory amino acid transporters (EAATs) are expressed throughout the central nervous system, where they are responsible for the reuptake of the excitatory neurotransmitter (S)-glutamate (Glu). (1) Recently, we have reported the discovery of the first subtype selective EAAT1 inhibitor 2-amino-4-(4-methoxyphenyl)-7-(naphthalen-1-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chrom ene-3-carbonitrile (UCPH-101) (1b) and presented an introductory structure-activity relationship (SAR) study. (2) Here, we present a detailed SAR by the design, synthesis, and pharmacological evaluation of analogues 1g-1t. By comparison of potencies of 1b, 1h, and 1i versus 1j, it is evident that potency is largely influenced by the chemical nature of the R(1) substituent. The study also demonstrates that any chemical change of the functional groups or a change to the parental scaffold results in the complete loss of inhibitory activity of the compounds at EAAT1. Finally, a bioavailability study of UCPH-101 determined the half-life to be 30 min in serum (rats) but also that it was not able to penetrate the blood-brain barrier to any significant degree.
Discovery of the first selective inhibitor of excitatory amino acid transporter subtype 1.[Pubmed:19161278]
J Med Chem. 2009 Feb 26;52(4):912-5.
The discovery of the first class of subtype-selective inhibitors of the human excitatory amino acid transporter subtype 1 (EAAT1) and its rat orthologue GLAST is reported. An opening structure-activity relationship of 25 analogues is presented that addresses the influence of substitutions at the 4- and 7-positions of the parental skeleton 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile. The most potent analogue 1o displays high nanomolar inhibitory activity at EAAT1 and a >400-fold selectivity over EAAT2 and EAAT3, making it a highly valuable pharmacological tool.


