Elastase InhibitorHNE inhibitor CAS# 111682-13-4 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111682-13-4 | SDF | Download SDF |
| PubChem ID | 5748191 | Appearance | Powder |
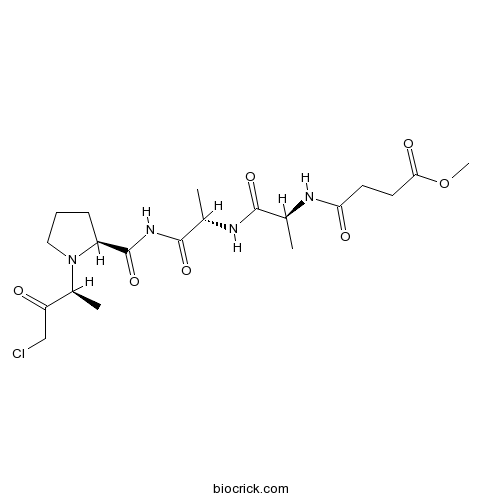
| Formula | C20H31ClN4O7 | M.Wt | 474.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | methyl 4-[[(2S)-1-[[(2S)-1-[[(2S)-1-[(2S)-4-chloro-3-oxobutan-2-yl]pyrrolidine-2-carbonyl]amino]-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-oxobutanoate | ||
| SMILES | CC(C(=O)NC(C)C(=O)NC(=O)C1CCCN1C(C)C(=O)CCl)NC(=O)CCC(=O)OC | ||
| Standard InChIKey | RHGXHACEVXSAHV-XUXIUFHCSA-N | ||
| Standard InChI | InChI=1S/C20H31ClN4O7/c1-11(22-16(27)7-8-17(28)32-4)18(29)23-12(2)19(30)24-20(31)14-6-5-9-25(14)13(3)15(26)10-21/h11-14H,5-10H2,1-4H3,(H,22,27)(H,23,29)(H,24,30,31)/t11-,12-,13-,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Elastase Inhibitor Dilution Calculator

Elastase Inhibitor Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1055 mL | 10.5276 mL | 21.0553 mL | 42.1106 mL | 52.6382 mL |
| 5 mM | 0.4211 mL | 2.1055 mL | 4.2111 mL | 8.4221 mL | 10.5276 mL |
| 10 mM | 0.2106 mL | 1.0528 mL | 2.1055 mL | 4.2111 mL | 5.2638 mL |
| 50 mM | 0.0421 mL | 0.2106 mL | 0.4211 mL | 0.8422 mL | 1.0528 mL |
| 100 mM | 0.0211 mL | 0.1053 mL | 0.2106 mL | 0.4211 mL | 0.5264 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A potent, irreversible inhibitor of human neutrophil elastase (HNE). The structure of the enzyme-inhibitor complex reveals crosslinking of the catalytic residues His-57 and Ser-195 by the peptidyl chloromethylketone.
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- Fmoc-D-Phg-OH
Catalog No.:BCC3316
CAS No.:111524-95-9
- Nyasicol
Catalog No.:BCN5999
CAS No.:111518-95-7
- Nyasicoside
Catalog No.:BCN5998
CAS No.:111518-94-6
- Metformin HCl
Catalog No.:BCC4799
CAS No.:1115-70-4
- L-Cysteinesulfinic acid
Catalog No.:BCC6571
CAS No.:1115-65-7
- H-Ala-OEt.HCl
Catalog No.:BCC2687
CAS No.:1115-59-9
- Ac-DL-Met-OH
Catalog No.:BCC2999
CAS No.:1115-47-5
- 25-Anhydroalisol F
Catalog No.:BCN3361
CAS No.:1114895-01-0
- Remacemide hydrochloride
Catalog No.:BCC7129
CAS No.:111686-79-4
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- BIM 23042
Catalog No.:BCC5998
CAS No.:111857-96-6
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
Identification and Characterization of Roseltide, a Knottin-type Neutrophil Elastase Inhibitor Derived from Hibiscus sabdariffa.[Pubmed:27991569]
Sci Rep. 2016 Dec 19;6:39401.
Plant knottins are of therapeutic interest due to their high metabolic stability and inhibitory activity against proteinases involved in human diseases. The only knottin-type proteinase inhibitor against porcine pancreatic elastase was first identified from the squash family in 1989. Here, we report the identification and characterization of a knottin-type human neutrophil Elastase Inhibitor from Hibiscus sabdariffa of the Malvaceae family. Combining proteomic and transcriptomic methods, we identified a panel of novel cysteine-rich peptides, roseltides (rT1-rT8), which range from 27 to 39 residues with six conserved cysteine residues. The 27-residue roseltide rT1 contains a cysteine spacing and amino acid sequence that is different from the squash knottin-type Elastase Inhibitor. NMR analysis demonstrated that roseltide rT1 adopts a cystine-knot fold. Transcriptome analyses suggested that roseltides are bioprocessed by asparagine endopeptidases from a three-domain precursor. The cystine-knot structure of roseltide rT1 confers its high resistance against degradation by endopeptidases, 0.2 N HCl, and human serum. Roseltide rT1 was shown to inhibit human neutrophil elastase using enzymatic and pull-down assays. Additionally, roseltide rT1 ameliorates neutrophil elastase-stimulated cAMP accumulation in vitro. Taken together, our findings demonstrate that roseltide rT1 is a novel knottin-type neutrophil Elastase Inhibitor with therapeutic potential for neutrophil elastase associated diseases.
A Plant Proteinase Inhibitor from Enterolobium contortisiliquum Attenuates Pulmonary Mechanics, Inflammation and Remodeling Induced by Elastase in Mice.[Pubmed:28216579]
Int J Mol Sci. 2017 Feb 14;18(2). pii: ijms18020403.
Proteinase inhibitors have been associated with anti-inflammatory and antioxidant activities and may represent a potential therapeutic treatment for emphysema. Our aim was to evaluate the effects of a plant Kunitz proteinase inhibitor, Enterolobium contortisiliquum trypsin inhibitor (EcTI), on several aspects of experimental elastase-induced pulmonary inflammation in mice. C57/Bl6 mice were intratracheally administered elastase (ELA) or saline (SAL) and were treated intraperitoneally with EcTI (ELA-EcTI, SAL-EcTI) on days 1, 14 and 21. On day 28, pulmonary mechanics, exhaled nitric oxide (ENO) and number leucocytes in the bronchoalveolar lavage fluid (BALF) were evaluated. Subsequently, lung immunohistochemical staining was submitted to morphometry. EcTI treatment reduced responses of the mechanical respiratory system, number of cells in the BALF, and reduced tumor necrosis factor-alpha (TNF-alpha), matrix metalloproteinase-9 (MMP-9), matrix metalloproteinase-12 (MMP-12), tissue inhibitor of matrix metalloproteinase (TIMP-1), endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS)-positive cells and volume proportion of isoprostane, collagen and elastic fibers in the airways and alveolar walls compared with the ELA group. EcTI treatment reduced elastase induced pulmonary inflammation, remodeling, oxidative stress and mechanical alterations, suggesting that this inhibitor may be a potential therapeutic tool for chronic obstructive pulmonary disease (COPD) management.
Neutrophil elastase inhibitor sivelestat ameliorates gefitinib-naphthalene-induced acute pneumonitis in mice.[Pubmed:28300553]
Biochem Biophys Res Commun. 2017 Apr 22;486(1):205-209.
Gefitinib, an epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI), is an effective therapeutic agent for non-small cell lung cancer with EGFR mutations. It can cause severe acute pneumonitis in some patients. We previously demonstrated that mice with naphthalene-induced airway epithelial injury developed severe gefitinib-induced pneumonitis and that neutrophils played important roles in the development of the disease. This study aimed to investigate the effects of the neutrophil Elastase Inhibitor sivelestat on gefitinib-induced pneumonitis in mice. C57BL/6J mice received naphthalene (200 mg/kg) intraperitoneally on day 0. Gefitinib (250 or 300 mg/kg) was orally administered to mice from day -1 until day 13. Sivelestat (150 mg/kg) was administered intraperitoneally from day 1 until day 13. Bronchoalveolar lavage fluid (BALF) and lung tissues were sampled on day 14. Sivelestat treatment significantly reduced the protein level, neutrophil count, neutrophil elastase activity in BALF, and severity of histopathologic findings on day 14 for mice administered with 250 mg/kg of gefitinib. Moreover, sivelestat treatment significantly improved the survival of mice administered with 300 mg/kg of gefitinib. These results indicate that sivelestat is a promising therapeutic agent for severe acute pneumonitis caused by gefitinib.
Study on Perioperative Administration of a Neutrophil Elastase Inhibitor for Interstitial Pneumonias.[Pubmed:28347540]
Ann Thorac Surg. 2017 Jun;103(6):1781-1787.
BACKGROUND: Although acute exacerbation of idiopathic interstitial pneumonias (IIPs) is a lethal complication after pulmonary resection for lung cancer with IIPs, there are no established methods to prevent its occurrence. This prospective randomized study was conducted to evaluate whether perioperative administration of the neutrophil Elastase Inhibitor sivelestat prevents acute exacerbation after surgery. METHODS: The IIP patients with suspected lung cancers were randomly assigned to two groups before surgery: in group A (n = 65), sivelestat was perioperatively administered for 5 days; in group B (n = 65), no medications were administered. The primary endpoint was the frequency of acute exacerbation of IIPs. The secondary endpoints were perioperative changes in the lactate dehydrogenase, C-reactive protein, sialylated carbohydrate antigen, surfactant protein D and surfactant protein A values, and the safety of preoperative administration of sivelestat. Multivariate analyses were performed using a logistic regression model to identify the factors that predicted acute exacerbation. RESULTS: Acute exacerbation developed in 2 patients in group A and 1 patient in group B (p = 0.559). Administration of sivelestat did not contribute to decreasing the acute exacerbation as well as short- and long-term mortality. The differences were not statistically significant in perioperative lactate dehydrogenase, C-reactive protein, sialylated carbohydrate antigen, and surfactant protein D and A levels. No subjective adverse events were observed. A preoperative partial pressure oxygen level of less than 70 mm Hg was the only predictive factor identified in the logistic analysis (p = 0.019, hazard ratio 19.2). CONCLUSIONS: Perioperative administration of neutrophil Elastase Inhibitor appeared to be safe; however, it could not prevent the development of acute exacerbation after surgery in lung cancer patients with IIPs.


