BIM 23042Antagonist of neuromedin B receptor,selective CAS# 111857-96-6 |
- CHIR-99021 (CT99021)
Catalog No.:BCC1275
CAS No.:252917-06-9
- SB 415286
Catalog No.:BCC3651
CAS No.:264218-23-7
- SB 216763
Catalog No.:BCC3650
CAS No.:280744-09-4
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111857-96-6 | SDF | Download SDF |
| PubChem ID | 118855703 | Appearance | Powder |
| Formula | C63H73N11O9S2 | M.Wt | 1192.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 0.1% acetic acid | ||
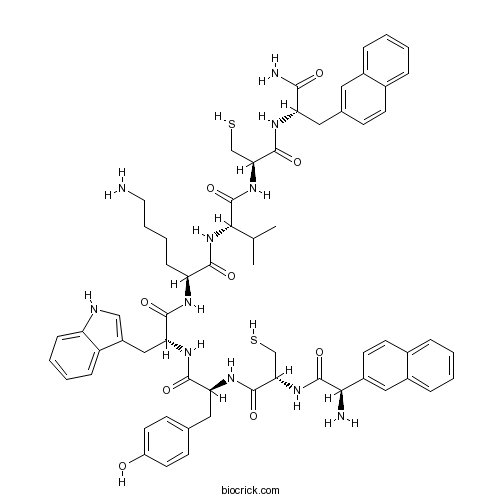
| Sequence | XCYWKVCX (Modifications: Disulfide bridge between 2 - 7, X-1 = D-Nal, Trp-4 = D-Trp, X-8 = Nal & C-terminal amide) | ||
| Chemical Name | (2S)-6-amino-2-[[(2R)-2-[[(2S)-2-[[(2R)-2-[[(2R)-2-amino-2-naphthalen-2-ylacetyl]amino]-3-sulfanylpropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-N-[(2S)-1-[[(2R)-1-[[(2S)-1-amino-3-naphthalen-2-yl-1-oxopropan-2-yl]amino]-1-oxo-3-sulfanylpropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]hexanamide | ||
| SMILES | CC(C)C(C(=O)NC(CS)C(=O)NC(CC1=CC2=CC=CC=C2C=C1)C(=O)N)NC(=O)C(CCCCN)NC(=O)C(CC3=CNC4=CC=CC=C43)NC(=O)C(CC5=CC=C(C=C5)O)NC(=O)C(CS)NC(=O)C(C6=CC7=CC=CC=C7C=C6)N | ||
| Standard InChIKey | AOVMHKJQNYSYAL-ADEFNUOKSA-N | ||
| Standard InChI | InChI=1S/C62H73N11O9S2/c1-35(2)54(62(82)72-52(34-84)59(79)68-48(55(65)75)29-37-18-21-38-11-3-5-13-40(38)27-37)73-56(76)47(17-9-10-26-63)67-58(78)50(31-43-32-66-46-16-8-7-15-45(43)46)70-57(77)49(28-36-19-24-44(74)25-20-36)69-60(80)51(33-83)71-61(81)53(64)42-23-22-39-12-4-6-14-41(39)30-42/h3-8,11-16,18-25,27,30,32,35,47-54,66,74,83-84H,9-10,17,26,28-29,31,33-34,63-64H2,1-2H3,(H2,65,75)(H,67,78)(H,68,79)(H,69,80)(H,70,77)(H,71,81)(H,72,82)(H,73,76)/t47-,48-,49-,50+,51-,52-,53+,54-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective neuromedin B receptor (NMB-R, BB1) antagonist (Ki values are 216 and 18,264 nM for BB1 and BB2 receptors respectively). Displays no activity at a range of other receptors. |

BIM 23042 Dilution Calculator

BIM 23042 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki: 49 ±14 nM for neuromedin B-induced endpoint in huNMBR cells
Neuromedin B, a mammalian peptide of the bombesinlike peptide family sharing amino acid homology with its amphibian counterpart ranatensin, elicits a diverse array of biological responses in central and peripheral tissues. BIM 23042 [D-Nal-Cys-Tyr- D-Trp-Lys-Val-Cys-Nal-NH2] is a selective neuromedin B antagonist.
In vitro: BIM 23042 has a l00-fold greater affinity for BB1 receptors than BB2 receptors. The submaximal mobilisation observed with neuromedin B (1 nM) was abolished by BIM 23042 but restored with a subsequently higher concentration of neuromedin B (1 μM). BIM 23042 competitively inhibited neuromedin B-induced endpoint in huNMBR cells with a Ki of 49 ±14 nM [1].
In vivo: In cat upper GI tract, SSocta, at concentrations of 10 mM, did not influence the smooth muscle tone but shifted NMB concentration response to the right yielding (Ki=1.7±0.8 mM). Ssocta inhibited both NMB- and GRP-induced contractions on the esophagus. the NMB-receptor antagonist SSocta had no effect on circular fundic muscle indicating the absence of this receptor subtype on fundus. [2].
Clinical trial: Up to now, BIM 23042 is still in the preclinical development stage.
References:
[1] Ryan RR, Taylor JE, Daniel JL, Cowan A. Pharmacological profiles of two bombesin analogues in cells transfected with human neuromedin B receptors. Eur J Pharmacol. 1996 Jun 13;306(1-3):307-14.
[2] Milusheva EA, Kortezova NI, Mizhorkova ZN, Papasova M, Coy DH, Bálint A, Vizi ES, Varga G. Role of different bombesin receptor subtypes mediating contractile activity in cat upper gastrointestinal tract. Peptides. 1998;19(3):549-56.
- UCPH 101
Catalog No.:BCC7692
CAS No.:1118460-77-7
- Hancinone C
Catalog No.:BCN4751
CAS No.:111843-10-8
- 10-O-Methylprotosappanin B
Catalog No.:BCN6599
CAS No.:111830-77-4
- (S)-(-)-HA-966
Catalog No.:BCC6589
CAS No.:111821-58-0
- H-Glu-Oet.HCl
Catalog No.:BCC2685
CAS No.:1118-89-4
- Demethoxyfumitremorgin C
Catalog No.:BCN7240
CAS No.:111768-16-2
- Remacemide hydrochloride
Catalog No.:BCC7129
CAS No.:111686-79-4
- Elastase Inhibitor
Catalog No.:BCC1225
CAS No.:111682-13-4
- GSK1838705A
Catalog No.:BCC3787
CAS No.:1116235-97-2
- Cyanidin-3-O-arabinoside chloride
Catalog No.:BCN3023
CAS No.:111613-04-8
- Anonamine
Catalog No.:BCN2139
CAS No.:111566-66-6
- 3',5-Dihydroxy-4',5',6,7-tetramethoxyflavone
Catalog No.:BCN1620
CAS No.:111537-41-8
- 2,4-Dihydroxyphenylacetyl-L-asparagine
Catalog No.:BCC6585
CAS No.:111872-98-1
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- H-Glu(OEt)-OH
Catalog No.:BCC2930
CAS No.:1119-33-1
- H-Arg-OH.HCl
Catalog No.:BCC2857
CAS No.:1119-34-2
- 2-Guanidinoethanesulfinic acid
Catalog No.:BCN1800
CAS No.:1119-54-6
- MitMAB
Catalog No.:BCC7892
CAS No.:1119-97-7
- (1S,3R)-ACPD
Catalog No.:BCC6590
CAS No.:111900-32-4
- Temocapril
Catalog No.:BCC5013
CAS No.:111902-57-9
- Adenanthin
Catalog No.:BCN6000
CAS No.:111917-59-0
- Quetiapine
Catalog No.:BCC1877
CAS No.:111974-69-7
- Quetiapine fumarate
Catalog No.:BCN5339
CAS No.:111974-72-2
- 2-Undecanone
Catalog No.:BCN8461
CAS No.:112-12-9
Pharmacological profiles of two bombesin analogues in cells transfected with human neuromedin B receptors.[Pubmed:8813645]
Eur J Pharmacol. 1996 Jun 13;306(1-3):307-14.
We examined the effect of two des-Met-bombesin analogues, [(CH3)2CHCO-His-Trp-Ala-Val-D-Ala-His-Leu-NHCH3] (ICI 216140) and [D-Phe6,des-Met14]bombesin(6-14) ethylamide (DPDM-bombesin ethylamide), on neuromedin B-induced Ca2+ and [3H]arachidonate release in BALB 3T3 cells transfected with human neuromedin B receptors. ICI 216140 and DPDM-bombesin ethylamide both stimulated Ca2+ mobilisation in a concentration-dependent manner but were less potent and efficacious than neuromedin B. BIM 23042 [D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Nal-NH2], a selective neuromedin B antagonist and [D-Arg1,D-Phe5,D-Trp7,9,Leu11]substance P, a broad-spectrum peptide receptor antagonist inhibited neuromedin B-, ICI 216140 and DPDM-bombesin ethylamide-induced Ca2+ release. Pretreatment of cells with either des-Met-bombesin analogue attenuated neuromedin B-induced Ca2+ elevations, suggesting similar agonist-sensitive Ca2+ pools. The pharmacological profiles revealed from the [3H]arachidonate assay were similar, although ICI 216140 was less potent and efficacious than DPDM-bombesin ethylamide. The data suggest that ICI 216140 and DPDM-bombesin ethylamide behave as agonists at the neuromedin B receptor, perhaps as a consequence of neuromedin B receptor overexpression.
Role of different bombesin receptor subtypes mediating contractile activity in cat upper gastrointestinal tract.[Pubmed:9533644]
Peptides. 1998;19(3):549-56.
Mammalian bombesin-like peptides, gastrin-releasing peptide (GRP) and neuromedin B (NMB) are known to increase the motility of different segments in the gut. The present study was carried out to identify the bombesin receptor subtypes mediating the contractions induced by exogenous bombesin-like peptides in muscle strips isolated from cat esophagus, fundus, and duodenum. Both GRP-10 and NMB evoked concentration-dependent contractions in circular strips of esophagus and fundus and in longitudinal strips of the duodenum. These contractions were tetrodotoxin- and atropine-resistant. The potency of NMB in esophageal strips was 33 times higher than that of GRP-10. The NMB-preferring receptor antagonists D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Nal-NH2 (SSocta) and D-Nal-cyclo[Cys-Tyr-D-Trp-Orn-Val-Cys]-Nal-NH2 (BIM-23127) shifted the NMB and GRP concentration-response curves to the right, while the GRP-preferring receptor antagonist [D-Phe6]Bombesin(6-13)-methyl-ester (BME) did not affect the response to the peptides. Isolated muscle strips from the cat fundus and duodenum showed a higher sensitivity to GRP-10 than to NMB. In both segments, BME shifted the GRP-10 and NMB concentration-response curves to the right, while SSocta had no effect. The antagonism of BME was competitive on duodenal but not competitive on fundic muscle. We conclude that the direct myogenic action of GRP-10 and NMB in the esophagus is mediated mainly via NMB-preferring receptors, while GRP-preferring receptors are responsible for the contractile responses to bombesin-like peptides in feline fundus and duodenum. Our data suggest that the GRP receptor population located on fundic muscle might be nonhomogeneous.
Discovery of a novel class of neuromedin B receptor antagonists, substituted somatostatin analogues.[Pubmed:7901752]
Mol Pharmacol. 1993 Oct;44(4):841-50.
Bombesin-related peptides have widespread activities in the central nervous system and peripheral tissues. Recent studies show two subtypes of receptors; a gastrin-releasing peptide (GRP) receptor subtype and a neuromedin B (NMB) receptor subtype exist. In contrast to the GRP receptor, no antagonists exist for the NMB receptor. In the present study we report that certain somatostatin (SS) octapeptide analogues function as selective NMB receptor antagonists. The most potent analogue, D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Nal-NH2, inhibited binding of 125I-[D-Tyr degree]NMB to NMB receptor-transfected 3T3 cells and C6 cells. This analogue had 100-fold lower affinity for GRP receptors. Structure-function studies were performed by synthesizing 18 structurally related SS octapeptide analogues; each of these analogues, but not native SS-14 or SS-28, also inhibited binding to NMB receptors. The stereochemistry at positions 1, 2, 7, and 8, the hydrophobicity and ring size of the substitution in positions 1, 3, and 4, and the basicity of the group in position 5 were all important in determining NMB receptor affinity. No SS octapeptide analogue increased [3H]inositol phosphates in NMB receptor-transfected cells; however, each analogue inhibited NMB-stimulated increases. The most potent analogue, D-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Nal-NH2, caused a parallel rightward shift of the NMB dose-response curve, the Schild plot slope was not significantly different from unity, and the affinity was 230 nM. SS octapeptide analogues also interacted with SS receptors and mu-opioid receptors; however, there was no correlation between the affinities of the analogues for these receptors and their affinities for NMB receptors, demonstrating that these activities can be separated. The results demonstrate for the first time a class of antagonists with > 100-fold selectivity for NMB versus GRP receptors. Because the structural requirements for determining NMB, SS, and mu-opioid receptor activity differ, it is likely that highly selective, specific, high affinity NMB receptor antagonists can now be developed that will be useful in defining the role of NMB in various physiological processes.


