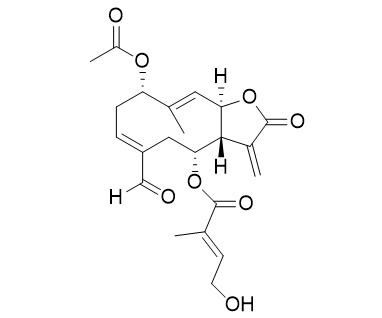
Eupalinolide OCAS# 2170228-67-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 2170228-67-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C22H26O8 | M.Wt | 418.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Eupalinolide O Dilution Calculator

Eupalinolide O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3901 mL | 11.9503 mL | 23.9006 mL | 47.8011 mL | 59.7514 mL |
| 5 mM | 0.478 mL | 2.3901 mL | 4.7801 mL | 9.5602 mL | 11.9503 mL |
| 10 mM | 0.239 mL | 1.195 mL | 2.3901 mL | 4.7801 mL | 5.9751 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.956 mL | 1.195 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Damulin B
Catalog No.:BCN8989
CAS No.:1202868-75-4
- Achyranthoside C
Catalog No.:BCN8988
CAS No.:168009-90-3
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Sibiricose A1
Catalog No.:BCN8991
CAS No.:139726-40-2
- Regaloside L
Catalog No.:BCN8992
CAS No.:
- Isorhamnetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN8993
CAS No.:60778-00-9
- Siaresinolic acid 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN8994
CAS No.:155653-86-4
- 2-(2-Phenylethyl)chromone
Catalog No.:BCN8995
CAS No.:61828-53-3
- Rheumone B
Catalog No.:BCN8996
CAS No.:2095596-67-9
- Gypenoside LI
Catalog No.:BCN8997
CAS No.:94987-10-7
- Hemiphloin
Catalog No.:BCN8998
CAS No.:71963-94-5
- Quercetagetinidin chloride
Catalog No.:BCN8999
CAS No.:42529-06-6
- Kaempferidinidin chloride
Catalog No.:BCN9000
CAS No.:13544-52-0
- Fisetinidin chloride
Catalog No.:BCN9001
CAS No.:2948-76-7
- Apigeninidin chloride
Catalog No.:BCN9002
CAS No.:1151-98-0
Precise discovery of a STAT3 inhibitor from Eupatorium lindleyanum and evaluation of its activity of anti-triple-negative breast cancer.[Pubmed:29086600]
Nat Prod Res. 2019 Feb;33(4):477-485.
Michael reaction acceptors (MRAs) are a class of active compounds. There is a great prospect to screen STAT3 inhibitors from Eupatorium lindleyanum, furthermore, to discover lead compounds for anti-triple-negative breast cancer (TNBC). In this study, glutathione (GSH) was employed, and a UPLC-MS screening method was developed to discover MRAs. We screened MRAs which can inhibit STAT3 using a STAT3-dependent reporter system. Six sesquiterpene lactones, including a new compound Eupalinolide O (1), together with five known compounds, Eupalinolide I (2), Eupalinolide K (3), Eupalinolide H (4), Eupalinolide J (5) and Eupalinolide G (6) were isolated. Eupalinolide J was identified as MRA that decreased luciferase activity of STAT3. Preliminary activity assessment showed that Eupalinolide J could inhibit the viability of TNBC cell lines. We demonstrated that Eupalinolide J, which is a natural typical MRA, has a notable inhibition of STAT3 activity and a potential cytotoxic activity against TNBC cell lines.
Eupalinolide O, a novel sesquiterpene lactone from Eupatorium lindleyanum DC., induces cell cycle arrest and apoptosis in human MDA-MB-468 breast cancer cells.[Pubmed:27666560]
Oncol Rep. 2016 Nov;36(5):2807-2813.
Sesquiterpene lactones have been confirmed to have potential antitumor activity. Here, we demonstrated that Eupalinolide O (EO), a novel sesquiterpene lactone isolated from Eupatorium lindleyanum DC., showed significant anticancer activity against human MDA-MB-468 breast cancer cells. The cytotoxicity induced by EO was mediated by induction of apoptosis. Flow cytometric analysis demonstrated that EO treatment resulted in loss of the mitochondrial membrane potential in cancer cells which is regarded as a hallmark of apoptosis. Further study demonstrated that EO induced apoptotic cell death in the MDA-MB-468 cells through the activation of caspases. The effect of EO on the induction of apoptosis was significantly prevented by the treatment of pan-caspase inhibitor Z-VAD-FMK. We also found that EO treatment resulted in cell cycle arrest in the G2/M phase. The expression of cell cycle-related proteins (cyclin B1 and cdc2) was significantly decreased. Furthermore, the suppression of the Akt pathway in the MDA-MB-468 cells was observed. Collectively, EO suppressed the growth of the MDA-MB468 cells possibly by cell cycle arrest in the G2/M phase and the induction of caspase-dependent apoptosis. These results suggest that EO is a promising natural compound for breast cancer therapy.


