PongamolCAS# 484-33-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 484-33-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
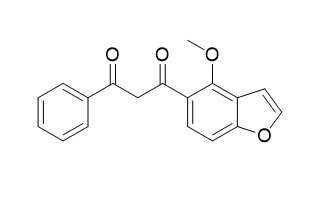
| Formula | C18H14O4 | M.Wt | 294.3 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Pongamol Dilution Calculator

Pongamol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3979 mL | 16.9895 mL | 33.9789 mL | 67.9579 mL | 84.9473 mL |
| 5 mM | 0.6796 mL | 3.3979 mL | 6.7958 mL | 13.5916 mL | 16.9895 mL |
| 10 mM | 0.3398 mL | 1.6989 mL | 3.3979 mL | 6.7958 mL | 8.4947 mL |
| 50 mM | 0.068 mL | 0.3398 mL | 0.6796 mL | 1.3592 mL | 1.6989 mL |
| 100 mM | 0.034 mL | 0.1699 mL | 0.3398 mL | 0.6796 mL | 0.8495 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
- Achyranthoside C
Catalog No.:BCN8988
CAS No.:168009-90-3
- Damulin B
Catalog No.:BCN8989
CAS No.:1202868-75-4
- Eupalinolide O
Catalog No.:BCN8990
CAS No.:2170228-67-6
- Sibiricose A1
Catalog No.:BCN8991
CAS No.:139726-40-2
- Regaloside L
Catalog No.:BCN8992
CAS No.:
- Isorhamnetin 3-O-beta-D-glucose-7-O-beta-D-gentiobioside
Catalog No.:BCN8993
CAS No.:60778-00-9
- Siaresinolic acid 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN8994
CAS No.:155653-86-4
- 2-(2-Phenylethyl)chromone
Catalog No.:BCN8995
CAS No.:61828-53-3
- Rheumone B
Catalog No.:BCN8996
CAS No.:2095596-67-9
- Gypenoside LI
Catalog No.:BCN8997
CAS No.:94987-10-7
- Hemiphloin
Catalog No.:BCN8998
CAS No.:71963-94-5
A comprehensive review on ethnomedicine, phytochemistry, pharmacology, and toxicity of Tephrosia purpurea (L.) Pers.[Pubmed:32147928]
Phytother Res. 2020 Mar 8.
Tephrosia purpurea (L.) Pers. is a well-known plant in Ayurveda and named "Sarwa wranvishapaka" for its property to heal wounds. Traditionally, it is practiced for impotency, asthma, dyspepsia, hemorrhoids, syphilis gonorrhea, rheumatism, enlargement of kidney and spleen. It is an important component of herbal preparations like Tephroli and Yakrifti used to cure liver disorders. Various phytocompounds including Pongamol, purpurin, purpurenone, tephrosin, bulnesol, tephrostachin, beta-sitosterol, and so on have been reported. Modern pharmacological studies have shown that the plant have wound healing, antileishmanial, anticarcinogenic, antimicrobial, antioxidant, hepatoprotective, antifertility, antispermatogenic, anti-diarrheal, diuretic, and insecticidal properties. Acetylcholinesterase inhibitory action reported from this plant aids its utilization for the development of drugs for Alzheimer's and dementia neurological disorders. Among the known active compounds of T. purpurea, tephrostachin is responsible for antiplasmodial activity, tephrosin, pongaglabol, and semiglabrin exerts antiulcer activity while quercetin, rutin, beta-sitosterol, and lupeol are mainly responsible for its anti-inflammatory and anti-cancer properties. From different toxicological studies, concentrations up to 2,000 mg/kg were considered safe. The present review comprehensively summarizes the ethnomedicine, phytochemistry, pharmacology, and toxicology of T. purpurea. Further research on elucidation of the structure-function relationship among active compounds, understanding of multi-target network pharmacology and clinical applications will intensify its therapeutic potential.
Furanoflavones pongapin and lanceolatin B blocks the cell cycle and induce senescence in CYP1A1-overexpressing breast cancer cells.[Pubmed:30448188]
Bioorg Med Chem. 2018 Dec 15;26(23-24):6076-6086.
Expression of cytochrome P450-1A1 (CYP1A1) is suppressed under physiologic conditions but is induced (a) by polycyclic aromatic hydrocarbons (PAHs) which can be metabolized by CYP1A1 to carcinogens, and (b) in majority of breast cancers. Hence, phytochemicals or dietary flavonoids, if identified as CYP1A1 inhibitors, may help in preventing PAH-mediated carcinogenesis and breast cancer. Herein, we have investigated the cancer chemopreventive potential of a flavonoid-rich Indian medicinal plant, Pongamia pinnata (L.) Pierre. Methanolic extract of its seeds inhibits CYP1A1 in CYP1A1-overexpressing normal human HEK293 cells, with IC50 of 0.6microg/mL. Its secondary metabolites, the furanoflavonoids pongapin/lanceolatin B, inhibit CYP1A1 with IC50 of 20nM. Although the furanochalcone Pongamol inhibits CYP1A1 with IC50 of only 4.4microM, a semisynthetic pyrazole-derivative P5b, has approximately 10-fold improved potency (IC50, 0.49muM). Pongapin/lanceolatin B and the methanolic extract of P. pinnata seeds protect CYP1A1-overexpressing HEK293 cells from B[a]P-mediated toxicity. Remarkably, they also block the cell cycle of CYP1A1-overexpressing MCF-7 breast cancer cells, at the G0-G1 phase, repress cyclin D1 levels and induce cellular-senescence. Molecular modeling studies demonstrate the interaction pattern of pongapin/lanceolatin B with CYP1A1. The results strongly indicate the potential of methanolic seed-extract and pongapin/lanceolatin B for further development as cancer chemopreventive agents.
Effects of flavonoids from Martynia annua and Tephrosia purpurea on cutaneous wound healing.[Pubmed:27761428]
Avicenna J Phytomed. 2016 Sep-Oct;6(5):578-591.
OBJECTIVE: Martynia annua L. (M. annua), (Martyniaccae) has been traditionally used in the treatment of epilepsy, sore throat and inflammatory disorders. The leaf paste is used topically on Tuberculosis of the lymphatic glands and wounds of domestic animals. Tephrosia purpurea (T. purpurea), (Fabaceae) has been used traditionally as a remedy for asthma, gonorrhea, rheumatism and ulcers. This study aimed to evaluate the potential wound healing effects of different fractions ofethanol extract of M. annua leaves and aerial parts of T. purpurea. MATERIALS AND METHODS: Methanol fraction of M. annua (MAF-C) and ethyl acetate fraction of T. purpurea (TPF-A) were evaluated for healing potential in dead-space and burn wound models. An ointment (5% w/w) of MAF-C and TPF-A, Pongamol (0.2 and 0.5% w/w) and luteolin (0.2 and 0.5% w/w) was applied topically twice a day. The effects were compared with Povidone Iodine ointment with respect to protein, collagen content, enzymatic assay and histopathological finding of granuloma tissues. RESULTS: Ethanol extracts of M. annua and T. purpureawere exhibited total flavonoid contents of 126.2 +/- 4.69 and 171.6 +/- 6.38 mg (quercetin equivalent), respectively. HPLC fingerprinting confirmed the presence of luteolin in M. annua and quercetin in T. purpurea. TPF-A and MAF-C ointments (5% w/w) significantly increases the hydroxyproline and protein contents. Luteolin and Pongamol ointments were also found to be effective in both wound models. CONCLUSION: Our findings suggested that 5% w/w ointment of TPF-A and MAF-C fractions were more effective than isolated flavonoids in wound healing which may be due to synergistic interactions between the flavonoids and other constituents.
An efficient transformation of furano-hydroxychalcones to furanoflavones via base mediated intramolecular tandem O-arylation and C-O bond cleavage: a new approach for the synthesis of furanoflavones.[Pubmed:26426474]
Org Biomol Chem. 2015 Nov 14;13(42):10461-5.
A new and efficient potassium carbonate mediated intramolecular tandem O-arylation followed by C-O bond cleavage of furano-hydroxychalcones is described. The treatment of furano-hydroxychalcones Pongamol (1a) and ovalitenone (2a) with potassium carbonate in DMF led to the direct formation of the furanoflavones lanceolatin B (3ab) and pongaglabrone (4ab) in excellent yields. This is the first report on the cyclization of furano-hydroxychalcones via C-O bond cleavage (demethoxylation) to produce furanoflavonoids.
Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species.[Pubmed:25928224]
Parasit Vectors. 2015 Apr 19;8:237.
BACKGROUND: Aedes aegypti and Aedes albopictus and Culex pipiens pallens mosquitoes transmit dengue fever and West Nile virus diseases, respectively. This study was conducted to determine the toxicity and mechanism of action of four flavonoids and two fatty acids from Millettia pinnata (Fabaceae) seed as well as six pure fatty acids and four fatty acid esters toward third instar larvae from insecticide-susceptible C. pipiens pallens and A. aegypti as well as wild A. albopictus. Efficacy of 12 experimental liquid formulations containing M. pinnata seed methanol extract and hydrodistillate (0.5-10.0% liquids) was also assessed. METHODS: The contact toxicities of all compounds and 12 formulations were compared with those of two larvicides, temephos and fenthion and the commercial temephos 200 g/L emulsifiable concentrate (EC). The possible mode of larvicidal action of the constituents was elucidated using biochemical methods. Larval mortality and cAMP level were analyzed by the Bonferroni multiple-comparison method. RESULTS: Potent toxicity was produced by karanjin, oleic acid, karanjachromene, linoleic acid, linolenic acid, Pongamol, pongarotene, and elaidic acid toward C. pipiens pallens larvae (24 h LC50, 14.61-28.22 mg/L) and A. aegypti larvae (16.13-37.61 mg/L). Against wild A. albopictus larvae, oleic acid (LC50, 18.79 mg/L) and karanjin (35.26 mg/L) exhibited potent toxicity. All constituents were less toxic than either temephos or fenthion. Structure-activity relationship indicates that the degree of saturation, the side chain length, and the geometric isomerism of fatty acids appear to play a role in determining the fatty acid toxicity. Acetylcholinesterase (AChE) is the main site of action of the flavonoids, oleic acid, and palmitic acid. The mechanism of larvicidal action of elaidic acid, arachidic acid, and behenic acid might be due to interference with the octopaminergic system. Linoleic acid and linolenic acid might act on both AChE and octopaminergic receptor. M. pinnata seed extract or hydrodistillate applied as 10% liquid provided 100% mortality toward the three mosquito species larvae and the efficacy of the liquids was comparable to that of temephos 200 g/L EC. CONCLUSION: Further studies will warrant possible applications of M. pinnata seed-derived products as potential larvicides for the control of mosquito populations.
A new 1,2-ethanedione benzofurane derivative from Tephrosia purpurea.[Pubmed:25116833]
Nat Prod Res. 2014;28(20):1705-8.
A new 1,2-ethanedione benzofurane derivative, purpdione (1), was isolated from Tephrosia purpurea, together with seven known flavonoids, purpurenone (2), Pongamol (3), ovalitenin A (4), karanjin (5), lanceolatin B (6), tachrosin (7) and villosinol (8). The new structure was elucidated based on the analysis of its spectroscopic data. The structures of the known compounds were identified by comparing their spectroscopic data with those reported in the literature. The isolates exhibited marginal ability to inhibit the settlement of barnacle (Balanus reticulatus).
Revisiting curcumin chemistry part I: a new strategy for the synthesis of curcuminoids.[Pubmed:22457548]
Indian J Pharm Sci. 2011 May;73(3):262-70.
A new strategy for the synthesis of curcuminoids is described involving the reaction of acetylacetone difluroboronite with an aromatic aldehyde in the presence of n-butylamine as catalyst. The new intermediate products, curcuminoid difluroboronites, of symmetrically substituted curcuminoids like curcumin and bisdemethoxycurcumin are stable, can be isolated and hydrolysed with aq. methanol at pH 5.8 to get the curcuminoids of high purity. The method is applicable for unsymmetrical curcuminoids like demethoxycurcumin also with some modification involving column chromatography. The intermediate curcuminoid difluroboronites, as also the natural beta-diketone Pongamol difluroboronite, prepared for the first time were characterized on the basis of physical and chemical properties and spectroscopic data. The advantage of using borontrifluoride to protect the enol group in acetylacetone over the generally used boric oxide is brought out. The importance of conducting biological activity studies using pure curcuminoids is explained.
Pongamol from Pongamia pinnata stimulates glucose uptake by increasing surface GLUT4 level in skeletal muscle cells.[Pubmed:21497640]
Mol Cell Endocrinol. 2011 Jun 6;339(1-2):98-104.
Skeletal muscle is the major site of postprandial glucose disposal and augmenting glucose uptake into this tissue may attenuate insulin resistance that precedes type 2 diabetes mellitus. Here, we investigated the effect of Pongamol, an identified lead molecule from the fruits of Pongamia pinnata, on glucose uptake and GLUT4 translocation in skeletal muscle cells. In L6-GLUT4myc myotubes treatment with Pongamol significantly promoted both glucose transport and GLUT4 translocation to the cell surface in a concentration-dependent manner, without changing the total amount of GLUT4 protein and GLUT4 mRNA, effects that were also additive with insulin. Cycloheximide treatment inhibited the effect of Pongamol on GLUT4 translocation suggesting the requirement of new protein synthesis. The Pongamol-induced increase in GLUT4 translocation was completely abolished by wortmannin, and Pongamol significantly potentiated insulin-mediated phosphorylation of AKT (Ser-473). We conclude that Pongamol-induced increase in glucose uptake in L6 myotubes is the result of an increased translocation of GLUT4 to plasma membrane, driven by a PI-3-K/AKT dependent mechanism.
New furanoflavanoids, intestinal alpha-glucosidase inhibitory and free-radical (DPPH) scavenging, activity from antihyperglycemic root extract of Derris indica (Lam.).[Pubmed:19515570]
Bioorg Med Chem. 2009 Jul 15;17(14):5170-5.
A bioassay-guided fractionation and chemical examination of antihyperglycemic root extract of Derris indica resulted in isolation and characterization of two new furanoflavanoids (1, 2) along with thirteen known compounds (3-15). Their structures were determined on the basis of extensive spectroscopic (IR, MS, 1D and 2D NMR) data analysis and by comparison with the literature data. All the compounds were tested in vitro for intestinal alpha-glucosidase inhibitory and DPPH radical activity. New compounds (1, 2) displayed moderate intestinal alpha-glucosidase inhibitory as well as free radical scavenging activity. Other compounds also displayed varying degrees of moderate intestinal alpha-glucosidase inhibitory activity. Pongamol (6) displayed potent intestinal alpha-glucosidase inhibition.
Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits.[Pubmed:18572336]
J Ethnopharmacol. 2008 Aug 13;118(3):435-9.
AIM OF THE STUDY: To identify Pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. MATERIAL AND METHODS: Streptozotocin-induced diabetic rats and hyperglycemic, hyperlipidemic and hyperinsulinemic db/db mice were used to investigate the antihyperglycemic activity of Pongamol and karangin isolated from the fruits of Pongamia pinnata. RESULTS: In streptozotocin-induced diabetic rats, single dose treatment of Pongamol and karanjin lowered the blood glucose level by 12.8% (p<0.05) and 11.7% (p<0.05) at 50mg /kg dose and 22.0% (p<0.01) and 20.7% (p<0.01) at 100mg/kg dose, respectively after 6h post-oral administration. The compounds also significantly lowered blood glucose level in db/db mice with percent activity of 35.7 (p<0.01) and 30.6 (p<0.01), respectively at 100mg/kg dose after consecutive treatment for 10 days. The compounds were observed to exert a significant inhibitory effect on enzyme protein tyrosine phosphatase-1B (EC 3.1.3.48). CONCLUSION: The results showed that Pongamol and karangin isolated from the fruits of Pongamia pinnata possesses significant antihyperglycemic activity in Streptozotocin-induced diabetic rats and type 2 diabetic db/db mice and protein tyrosine phosphatase-1B may be the possible target for their activity.
Flavonoids of Lonchocarpus montanus A.M.G. Azevedo and biological activity.[Pubmed:17768528]
An Acad Bras Cienc. 2007 Sep;79(3):351-67.
The analysis of root extracts from Lonchocarpus montanus A.M.G. Azevedo resulted in the isolation of twenty three compounds chiefly flavonoids of which five (four flavonoids and one benzophenone) are described for the first time. The molecular structures of the new compounds (1-5) were determined through spectral analysis (UV, IR, MS and NMR) as being: 2'-hydroxy-8-(alpha,alpha-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (1), 2'-methoxy-8-(alpha,alpha-dimethylallyl)-2", 2"-dimethylpyrano-(5",6":3',4')-dibenzoylmethane (2), 4'-methoxy-2",2"-dimethylpyrano-(5",6":8,7)-flavone (3), 2"-(1-hydroxy-1-methylethyl)-furano-(4",5":8,7)-flavone (4) and [2'-methoxy-furano-(4",5":3',4')-phenyl]-phenylmethanone (5). Additionally, fifteen fatty acids were detected through GC-MS analysis of the corresponding methyl esters [(CH3)2CH(CH2)8COOH and CH3(CH2)nCOOH (n = 6, 12-24)]. Quantitative RP-HPLC showed that the most abundant flavonoids in the petroleum ether and dichloromethane extracts were Pongamol (19%) and lanceolatine B (8.0%), respectively. In the bioautography assay, the extracts, Pongamol (9), lanceolatine B (10), isolonchocarpin (14), derriobtusone A (17) and medicarpine (18) were active against Staphylococcus aureus whereas 9 also against Bacillus subtilis and Cladosporium cladosporioides. Compound 1, 2",2"-dimethylpyrano-(5",6":8,7)-flavone (11) and furano-(1200,1300:7,8)- 4'-methoxy flavone (12) were active against Fusarium oxysporium whereas 11 also against Rhizopus orizae. The extracts, compounds 9, 10, 17 and (E)-7-O-methoxyPongamol (23) displayed high toxicity in the brine shrimp lethality assay.
Activity-guided isolation of constituents of Tephrosia purpurea with the potential to induce the phase II enzyme, quinone reductase.[Pubmed:9322358]
J Nat Prod. 1997 Sep;60(9):869-73.
An isoflavone, 7,4'-dihydroxy-3',5'-dimethoxyisoflavone (1), and a chalcone, (+)-tephropurpurin (2), both novel compounds, as well as six constituents of known structure, (+)-purpurin (3), Pongamol (4), lanceolatin B (5), (-)-maackiain (6), (-)-3-hydroxy-4-methoxy-8,9-methylene-dioxypterocarpan (7), and (-)-medicarpin (8), were obtained as active compounds from Tephrosia purpurea, using a bioassay based on the induction of quinone reductase (QR) activity with cultured Hepa 1c1c7 mouse hepatoma cells. Additionally, three inactive compounds of known structure, 3'-methoxydaidzein, desmoxyphyllin B, and 3,9-dihydroxy-8-methoxycoumestan, were isolated and identified. The structure elucidation of compounds 1 and 2 was carried out by spectral data interpretation.


