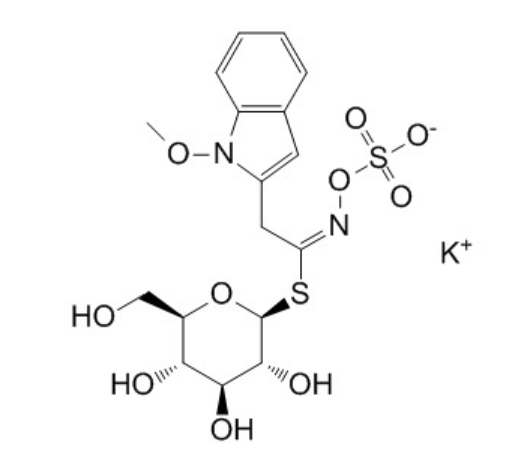
Neoglucobrassicin potassium saltCAS# 5187-84-8 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 5187-84-8 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C17H21KN2O10S2 | M.Wt | 516.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Neoglucobrassicin potassium salt Dilution Calculator

Neoglucobrassicin potassium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9357 mL | 9.6787 mL | 19.3573 mL | 38.7147 mL | 48.3933 mL |
| 5 mM | 0.3871 mL | 1.9357 mL | 3.8715 mL | 7.7429 mL | 9.6787 mL |
| 10 mM | 0.1936 mL | 0.9679 mL | 1.9357 mL | 3.8715 mL | 4.8393 mL |
| 50 mM | 0.0387 mL | 0.1936 mL | 0.3871 mL | 0.7743 mL | 0.9679 mL |
| 100 mM | 0.0194 mL | 0.0968 mL | 0.1936 mL | 0.3871 mL | 0.4839 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
- Achyranthoside C
Catalog No.:BCN8988
CAS No.:168009-90-3
- Damulin B
Catalog No.:BCN8989
CAS No.:1202868-75-4
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Use of elicitation in the cultivation of Bimi(R) for food and ingredients.[Pubmed:31875967]
J Sci Food Agric. 2020 Mar 30;100(5):2099-2109.
BACKGROUND: Cruciferous foods rich in health-promoting metabolites are of particular interest to consumers as well as being a good source of bioactives-enriched ingredients. Several elicitors have been used to stimulate the biosynthesis and accumulation of secondary metabolites in foods; however, little is known about the response of new hybrid varieties, such as Bimi(R), under field-crop production conditions. Therefore, this study was designed to evaluate the effect of salicylic acid (200 mumol L(-1) , SA), methyl jasmonate (100 mumol L(-1) , MeJA), and their combination on Bimi plant organs (inflorescences and aerial vegetative tissues - stems and leaves). For this, the composition of the glucosinolates present in the tissues was evaluated. Also, aqueous extracts of the plant material, obtained with different times of extraction with boiling water, were studied. RESULTS: The results indicate that the combined treatment (SA + MeJA) significantly increased the content of glucosinolates in the inflorescences and that MeJA was the most effective elicitor in leaves. Regarding the aqueous extracts, the greatest amount of glucosinolates was extracted at 30 min - except for the leaves elicited with MeJA, for which 15 min was optimal. CONCLUSION: The elicitation in the field enriched leaves in glucobrassicin (GB), 4-methoxyglucobrassicin (MGB), and neoglucobrassicin (NGB) and stems and inflorescences in glucoraphanin, 4-hydroxyglucobrassicin, GB, MGB, and NGB. In this way, this enhanced vegetable material favored the presence of bioactives in the extracts, which is of great interest regarding enriched foods and ingredients with added value obtained from them. (c) 2019 Society of Chemical Industry.
Methyl jasmonate treated broccoli: Impact on the production of glucosinolates and consumer preferences.[Pubmed:31299513]
Food Chem. 2019 Nov 30;299:125099.
Applying methyl jasmonate can mimic the defense response to insect damage in broccoli and enhances the production of glucosinolates, especially inducible indolyl GS-neoglucobrassicin. Previous studies have suggested that glucosinolates and their hydrolysis products are anti-carcinogenic. Therefore, MeJA treatment may increase the nutritional quality of broccoli. However, there are few reports on the sensory evaluation and consumer acceptance of MeJA-treated broccoli. In this study, an untrained consumer panel could not detect any taste differences between steamed MeJA-treated and untreated broccoli, even though the steamed MeJA-treated broccoli contained 50% more glucosinolates than untreated broccoli. The partial least square-regression model suggested that neoglucobrassicin-derived hydrolysis compounds were the major metabolites that determined overall preference for raw MeJA-treated broccoli potentially due to their potential negative sensory qualities. The results imply that MeJA treatment can increase the nutritional quality of broccoli without sacrificing taste in precooked meals or frozen vegetables.
Glucosinolate Content and Sensory Evaluation of Baby Leaf Rapeseed from Annual and Biennial White- and Yellow-Flowering Cultivars with Repeated Harvesting in Two Seasons.[Pubmed:31237979]
J Food Sci. 2019 Jul;84(7):1888-1899.
The chemical and sensory quality of field-grown vegetables may be influenced by cultivar choice and agronomic factors but knowledge is lacking on the new rapeseed vegetables. White- and yellow-flowering rapeseed cultivars were tested in two seasonally different field studies in Denmark at three different growing stages by early sowing the first year and late sowing the second year. Content of glucosinolates (GLSs) was analyzed, and the sensory quality of baby leaf samples was evaluated. The GLS content differed among cultivars across years in all growing stages, with biennial cultivars having the highest GLS content. In the second year, a higher content of all identified GLSs was found at two growing stages except for neoglucobrassicin and gluconasturtiin, compared to the first year. On the contrary, higher contents of all identified GLSs were found at a third stage in the first year except for progoitrin and 4-methoxy glucobrassicin. Sensory evaluation of bitterness revealed differences among cultivars, higher intensities of bitterness in biennial cultivars, and a relationship between bitterness and content of bitter-tasting and total GLSs. The effect of repeated harvesting on GLS content differed between the years and no general pattern was seen, except that the composition of individual GLSs was comparable for the biennial cultivars. We conclude that growing season and life cycle had a stronger influence on GLS content than stage at harvest. The link between bitter-tasting GLSs and bitterness revealed that life cycle and seasonal effects affected the sensory profile of baby leaf rapeseed thereby making a healthier product due to high content of health-beneficial GLSs.
Comparative analysis of glucosinolate production in hairy roots of green and red kale (Brassica oleracea var. acephala).[Pubmed:31124740]
Prep Biochem Biotechnol. 2019;49(8):775-782.
Glucosinolates (GSLs) are sulfur- and nitrogen-containing secondary metabolites that function in plant defense and provide benefits to human health. In this study, using Agrobacterium rhizogenes R1000, green and red kale hairy roots were established. The expression levels of GSLs biosynthesis genes and their accumulation in both kale hairy roots were analyzed by quantitative real-time PCR and HPLC. The results showed that the expression of most indolic GSLs biosynthesis genes was higher in the hairy roots of green kale than in that of red kale. In contrast, the expression of BoCYP83A1 and BoSUR1 encoding key enzymes aromatic GSL biosynthesis was significantly higher in red kale hairy root. The HPLC analysis identified six GSLs. The levels of 4-methoxyglucobrassicin, glucobrassicin, and 4-hydroxyglucobrassicin were 6.21, 5.98, and 2 times higher, respectively, in green kale than in red kale, whereas the levels of neoglucobrassicin and gluconasturtiin were 16.2 and 3.48 times higher, respectively, in red kale than in green kale. Our study provides insights into the underlying mechanisms of GSLs biosynthesis in kale hairy roots and can be potentially used as "biological factories" for producing bioactive substances such as GSLs.
Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets.[Pubmed:30274889]
Ultrason Sonochem. 2019 Jan;50:289-301.
Postharvest treatments such as wounding, ultrasound (US) and the exogenous application of ethylene (ET) and methyl jasmonate (MJ) have been studied as an effective tool to improve the content of secondary metabolites in fresh produce. The present study evaluated the immediate and late response (storage for 72h at 15 degrees C) to US treatment (20min, frequency 24kHz, amplitude 100mum) alone and combined with exogenous MJ (250ppm) and/or ET (1000ppm) on glucosinolates, isothiocyanates, phenolic compounds and ascorbic acid content in broccoli florets. US treatment increased the extractability of glucosinolates [glucoraphanin (795%), 4-hydroxy glucobrassicin (153%), glucobrassicin (78.6%)] and phenolics [1-sinapoyl-2-feruloylgentiobiose (57.23%)] as compared with the control (CT). The combined application of MJ and US in broccoli florets, induced a synergistic effect on the accumulation of 4-hydroxy glucobrassicin (187.1%), glucoerucin (111.92%), gluconasturtiin (755.9%), neoglucobrassicin (232.8%), 3-O-caffeoylquinic acid (73.4%), 1-sinapoyl-2-ferulolylgentiobiose (56.0%), and 1,2,2-trisinapoylgentiobiose (136.7%) at 72h of storage. Interestingly, when the three stressors were applied together the synergistic effect of US+MJ observed on the accumulation of glucosinolates and phenolics was repressed. In general, the ascorbic acid content was not affected by US treatment and decreased in most samples during storage. However, when MJ+ET were applied, the content of total ascorbic acid was significantly reduced in CT+MJ+ET and US+MJ+ET samples after 72h of storage by 53.4% and 86.6%, respectively, as compared with CT 0h samples. Based on the results herein obtained, the application of US can be an effective tool to enhance the extractability of certain glucocosinolate and phenolic compounds in broccoli. Moreover, due to the synergistic effect observed on the accumulation of bioactive compounds, the combined application of US and MJ could be a practical approach to yield higher levels of glucosinolates and phenolic compounds in broccoli during storage.
Comparative analysis of glucosinolates and metabolite profiling of green and red mustard (brassica juncea) hairy roots.[Pubmed:30148032]
3 Biotech. 2018 Sep;8(9):382.
Here, accumulation of glucosinolates and expression of glucosinolates biosynthesis genes in green and red mustard hairy roots were identified and quantified by HPLC and qRT-PCR analyses. The total glucosinolates content of green mustard hairy root (10.09 microg/g dry weight) was 3.88 times higher than that of red mustard hairy root. Indolic glucosinolates (glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin) in green mustard were found at 30.92, 6.95, and 5.29 times higher than in red mustard hairy root, respectively. Conversely, levels of glucotropaeolin (aromatic glucosinolate) was significantly higher in red mustard than in green mustard. Accumulation of glucoraphasatin, an aliphatic glucosinolate, was only observed only in red mustard hairy roots. Quantitative real-time PCR analysis showed that the expression level of genes related to aliphatic and aromatic glucosinolate biosynthesis were higher in red mustard, exception BjCYP83B. The expression of BjCYP79B2, which encodes a key enzyme involved in the indolic glucosinolate biosynthetic pathway, was higher in green mustard than in red mustard. Additionally, to further distinguish between green mustard and red mustard hairy roots, hydrophilic and lipophilic compounds were identified by gas chromatography-mass spectrometry and subjected to principal component analysis. The results indicated that core primary metabolites and glucosinolate levels were higher in the hairy roots of green mustard than in those of red mustard.
Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa.[Pubmed:30056602]
Bioprocess Biosyst Eng. 2018 Nov;41(11):1665-1677.
Glucosinolates (GSLs) and phenolic compounds (PCs) are biologically active and involved in the defense reaction of plants; these compounds have a beneficial effect on human health. In this study, we described the influence of biologically synthesized silver nanoparticles (Ag NPs) to enhance the phytochemicals (GSLs and PCs), their transcription levels, and their biological activities in genetically transformed root cultures (hairy root cultures) of Brassica rapa. The concentrations of silver and reactive oxygen species (malondialdehyde and hydrogen peroxide) were highly elevated in the Ag NP-elicited hairy roots (HRs). Glucosinolates (glucoallysin, glucobrassicanapin, sinigrin, progoitrin, gluconapin, 4-methoxyglucobrassicin, 4-hydroxyglucobrassicin, glucobrassicin, neoglucobrassicin, and gluconasturtiin) and their transcripts (MYB34, MYB51, MYB28, and MYB29) were significantly enhanced in the Ag NP-elicited HRs. Moreover, the phenolic compounds (flavonols, hydroxybenzoic, and hydroxycinnamic acids) were significantly enriched in the Ag NP-elicited HRs. Total phenolic and flavonoid concentrations and their transcripts (PAL, CHI, and FLS) were higher in the Ag NP-elicited HRs than in the non-elicited HRs. Additionally, biological (antioxidant, antimicrobial, and anticancer) activities were significantly higher in the Ag NP-elicited HRs than in the non-elicited HRs. The Ag NP-elicited HR cultures offered an efficient and promising in vitro method to increase the production of health-promoting bioactive compounds, which may be useful in nutraceutical and pharmaceutical industries.
Comparative Phytonutrient Analysis of Broccoli By-Products: The Potentials for Broccoli By-Product Utilization.[Pubmed:29652847]
Molecules. 2018 Apr 13;23(4). pii: molecules23040900.
The phytonutrient concentrations of broccoli (Brassica oleracea var. italica) florets, stems, and leaves were compared to evaluate the value of stem and leaf by-products as a source of valuable nutrients. Primary metabolites, including amino acids, organic acids, and sugars, as well as glucosinolates, carotenoids, chlorophylls, vitamins E and K, essential mineral elements, total phenolic content, antioxidant activity, and expression of glucosinolate biosynthesis and hydrolysis genes were quantified from the different broccoli tissues. Broccoli florets had higher concentrations of amino acids, glucoraphanin, and neoglucobrassicin compared to other tissues, whereas leaves were higher in carotenoids, chlorophylls, vitamins E and K, total phenolic content, and antioxidant activity. Leaves were also good sources of calcium and manganese compared to other tissues. Stems had the lowest nitrile formation from glucosinolate. Each tissue exhibited specific core gene expression profiles supporting glucosinolate metabolism, with different gene homologs expressed in florets, stems, and leaves, which suggests that tissue-specific pathways function to support primary and secondary metabolic pathways in broccoli. This comprehensive nutrient and bioactive compound profile represents a useful resource for the evaluation of broccoli by-product utilization in the human diet, and as feedstocks for bioactive compounds for industry.
Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis).[Pubmed:29379360]
Saudi J Biol Sci. 2018 Jan;25(1):71-82.
The present study aimed to investigate the contents of glucosinolates (GSLs) and carotenoids in eleven varieties of Chinese cabbage in relation to the expression level of the important transcription factors. MS and HPLC analysis identified the presence of 13 GSLs (progoitrin, sinigrin, glucoalyssin, gluconapoleiferin, gluconapin, glucocochlearin, glucobrassicanapin, glucoerucin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin and gluconasturtiin) and four carotenoids (lutein, zeaxanthin, alpha-carotene and beta-carotene). GSL contents were varied among the different cabbage varieties. The total GSL content ranged from 2.7 to 57.88 mumol/g DW. The proportion of gluconapin (54%) and glucobrassicanapin (22%) was higher in all the varieties, respectively. Results documented the variation in total and individual carotenoid contents that have also been observed among different varieties; however, the total carotenoid contents ranged from 289.12 to 1001.41 mg kg(-1) DW (mean 467.66). Interestingly, the proportion of lutein (66.5) and beta-carotene (25.9) were higher than alpha-carotene (5.1) and zeaxanthin (2.5%). Consequently, the expression level of the regulatory gene, MYB28 was higher in 'K0648' and was directly proportional to GSL content. Similarly, the expression levels of 1-PSY were higher in 'K0112'; however, the expression levels of 2-ZDS, 3-LCYB, 4-LCYE, 5-CHXB and 7-NCED genes showed no significant difference. In addition, the correlation between GSL and carotenoid contents and gene expression level showed moderate significant difference in each Chinese cabbage.
Synthesis of aromatic and indole alpha-glucosinolates.[Pubmed:29169042]
Carbohydr Res. 2018 Jan 2;455:45-53.
Aromatic and indole glucosinolates are important members of the glucosinolate family of compounds du to their potential medicinal properties. They are known to exert antioxidant and anti-carcinogenic activity either by the natural products themselves, or their metabolic products including indole-3-carbinol and isothiocyanates. Natural glucosinolates are all beta-glucosinolates; however, alpha-glucosinolates are also promising compounds for medicinal applications and hence have to be produced synthetically for any bio-activity studies. Here we report on the successful synthesis of a series of alpha-glucosinolates: alpha-neoglucobrassicin, alpha-4-methoxyglucobrassicin, 2,3-dichlorophenyl-alpha-glucosinolate for the first time. Testing for anti-inflammatory properties of these synthetic GLs, however, did not yield the expected activity.
Rapid and Efficient Desulfonation Method for the Analysis of Glucosinolates by High-Resolution Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry.[Pubmed:29161816]
J Agric Food Chem. 2017 Dec 20;65(50):11100-11108.
The goal of our present research was to develop a simple and rapid method for the quantitation of desulfoglucosinolates (desulfoGLS) without using column chromatography. The proposed method involves extraction, concentration, incubation of glucosinolates with a sulfatase enzyme, and HPLC analysis. Identification of desulfoGLS in green kohlrabi was performed by LC-HR-ESI-QTOF-MS in positive-ionization mode. A total of 11 desulfoGLS were identified with neoglucobrassicin (3.32 +/- 0.05 mumol/g DW) as the predominant indolyl, whereas progoitrin and sinigrin were the major aliphatic desulfoGLS. The levels of the aliphatic desulfoGLS glucoiberin, progoitrin, and glucoerucin at 7 h were found to be 3.6-, 1.9-, and 1.6-fold higher, respectively, than those produced through the conventional method. This technique was successfully applied in the identification of desulfoGLS from cabbage. The developed method has fewer unit operations, has maximum recovery, and is reproducible in the determination of desulfoGLS in a large number of Brassicaceae samples in a short time.
UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts.[Pubmed:29113068]
Int J Mol Sci. 2017 Nov 4;18(11). pii: ijms18112330.
Broccoli sprouts contain health-promoting phytochemicals that can be enhanced by applying ultraviolet light (UV) or phytohormones. The separate and combined effects of methyl jasmonate (MJ), UVA, or UVB lights on glucosinolate, phenolic, carotenoid, and chlorophyll profiles were assessed in broccoli sprouts. Seven-day-old broccoli sprouts were exposed to UVA (9.47 W/m(2)) or UVB (7.16 W/m(2)) radiation for 120 min alone or in combination with a 25 microM MJ solution, also applied to sprouts without UV supplementation. UVA + MJ and UVB + MJ treatments increased the total glucosinolate content by ~154% and ~148%, respectively. MJ induced the biosynthesis of indole glucosinolates, especially neoglucobrassicin (~538%), showing a synergistic effect with UVA stress. UVB increased the content of aliphatic and indole glucosinolates, such as glucoraphanin (~78%) and 4-methoxy-glucobrassicin (~177%). UVA increased several phenolics such as gallic acid (~57%) and a kaempferol glucoside (~25.4%). MJ treatment decreased most phenolic levels but greatly induced accumulation of 5-sinapoylquinic acid (~239%). MJ treatments also reduced carotenoid and chlorophyll content, while UVA increased lutein (~23%), chlorophyll b (~31%), neoxanthin (~34%), and chlorophyll a (~67%). Results indicated that UV- and/or MJ-treated broccoli sprouts redirect the carbon flux to the biosynthesis of specific glucosinolates, phenolics, carotenoids, and chlorophylls depending on the type of stress applied.


