LupinineCAS# 486-70-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

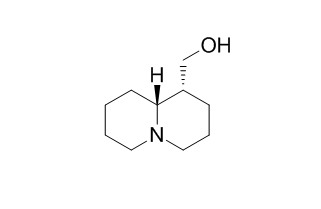
| Cas No. | 486-70-4 | SDF | Download SDF |
| PubChem ID | 91461 | Appearance | Powder |
| Formula | C10H19NO | M.Wt | 169.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,9aR)-2,3,4,6,7,8,9,9a-octahydro-1H-quinolizin-1-yl]methanol | ||
| SMILES | C1CCN2CCCC(C2C1)CO | ||
| Standard InChIKey | HDVAWXXJVMJBAR-VHSXEESVSA-N | ||
| Standard InChI | InChI=1S/C10H19NO/c12-8-9-4-3-7-11-6-2-1-5-10(9)11/h9-10,12H,1-8H2/t9-,10+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lupinine Dilution Calculator

Lupinine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.9067 mL | 29.5334 mL | 59.0667 mL | 118.1335 mL | 147.6669 mL |
| 5 mM | 1.1813 mL | 5.9067 mL | 11.8133 mL | 23.6267 mL | 29.5334 mL |
| 10 mM | 0.5907 mL | 2.9533 mL | 5.9067 mL | 11.8133 mL | 14.7667 mL |
| 50 mM | 0.1181 mL | 0.5907 mL | 1.1813 mL | 2.3627 mL | 2.9533 mL |
| 100 mM | 0.0591 mL | 0.2953 mL | 0.5907 mL | 1.1813 mL | 1.4767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
Phytochemical Information and Biological Activities of Quinolizidine Alkaloids in Sophora: A Comprehensive Review.[Pubmed:31215388]
Curr Drug Targets. 2019;20(15):1572-1586.
Quinolizidine alkaloids, a main form of alkaloids found in the genus Sophora, have been shown to have many pharmacological effects. This review aims to summarize the photochemical reports and biological activities of quinolizidine alkaloids in Sophora. The collected information suggested that a total of 99 quinolizidine alkaloids were isolated and detected from different parts of Sophora plants, represented by Lupinine-type, cytisine-type, sparteine-type, and matrine-type. However, quality control needs to be monitored because it could provide basic information for the reasonable and efficient use of quinolizidine alkaloids as medicines and raw materials. The nonmedicinal parts may be promising to be used as a source of quinolizidine alkaloid raw materials and to reduce the waste of resources and environmental pollution. In addition, the diversity of chemical compounds based on the alkaloid scaffold to make a biological compound library needs to be extended, which may reduce toxicity and find new bioactivities of quinolizidine alkaloids. The bioactivities most reported are in the fields of antitumor activity along with the effects on the cardiovascular system. However, those studies rely on theoretical research, and novel drugs based on quinolizidine alkaloids are expected.
Discovery of Natural Compounds Promoting Cardiomyocyte Differentiation.[Pubmed:30358491]
Stem Cells Dev. 2019 Jan 1;28(1):13-27.
The commitment of pluripotent stem cells to the cardiac lineage has enormous potential in regenerative medicine interventions for several cardiac diseases. Thus, it is necessary to understand and regulate this differentiation process for potential clinical application. In this study, we developed defined conditions with chemical inducers for effective cardiac lineage commitment and elucidated the mechanism for high-efficiency differentiation. First, we designed a robust reporter-based platform to screen chemical inducers of cardiac differentiation in the mouse P19 teratocarcinoma cell line. Using this system, we identified two natural alkaloids, Lupinine and ursinoic acid, which enhanced cardiomyocyte differentiation of P19 cells in terms of beating colony numbers with respect to oxytocin, and confirmed their activity in mouse embryonic stem cells. By analyzing the expression of key markers, we found that this enhancement can be attributed to the early and rapid induction of the Wnt signaling pathway. We also found that these natural compounds could not only supersede the action of the Wnt3a ligand but also had a very quick response time, allowing them to act as efficient cardiac mesoderm inducers that subsequently promoted cardiomyocyte differentiation. Thus, this study offers a way to develop chemical-based differentiation strategy for high-efficiency cardiac lineage commitment, which has an advantage over currently available methods with complex medium composition and parameters. Furthermore, it also provides an opportunity to pinpoint the key molecular mechanisms pivotal to the cardiac differentiation process, which are necessary to design an efficient strategy for cardiomyocyte differentiation.
In Vivo and In Vitro Activities and ADME-Tox Profile of a Quinolizidine-Modified 4-Aminoquinoline: A Potent Anti-P. falciparum and Anti-P. vivax Blood-Stage Antimalarial.[Pubmed:29194347]
Molecules. 2017 Dec 1;22(12). pii: molecules22122102.
Natural products are a prolific source for the identification of new biologically active compounds. In the present work, we studied the in vitro and in vivo antimalarial efficacy and ADME-Tox profile of a molecular hybrid (AM1) between 4-aminoquinoline and a quinolizidine moiety derived from Lupinine (Lupinus luteus). The aim was to find a compound endowed with the target product profile-1 (TCP-1: molecules that clear asexual blood-stage parasitaemia), proposed by the Medicine for Malaria Venture to accomplish the goal of malaria elimination/eradication. AM1 displayed a very attractive profile in terms of both in vitro and in vivo activity. By using standard in vitro antimalarial assays, AM1 showed low nanomolar inhibitory activity against chloroquine-sensitive and resistant P. falciparum strains (range IC50 16-53 nM), matched with a high potency against P. vivax field isolates (Mean IC50 29 nM). Low toxicity and additivity with artemisinin derivatives were also demonstrated in vitro. High in vivo oral efficacy was observed in both P.berghei and P. yoelii mouse models with IC50 values comparable or better than those of chloroquine. The metabolic stability in different species and the pharmacokinetic profile in the mouse model makes AM1 a compound worth further investigation as a potential novel schizonticidal agent.
The asymmetric syntheses of pyrrolizidines, indolizidines and quinolizidines via two sequential tandem ring-closure/N-debenzylation processes.[Pubmed:25300749]
Org Biomol Chem. 2014 Dec 7;12(45):9223-35.
Concise asymmetric syntheses of (-)-Lupinine, (+)-isoretronecanol, (+)-5-epi-tashiromine and (R,R)-1-(hydroxymethyl)octahydroindolizine (the azabicyclic core within stellettamides A-C) have been achieved in 8 steps or fewer from commercially available starting materials. The key steps in these syntheses involved the preparation of enantiopure beta-amino esters, upon conjugate addition of lithium (R)-N-(p-methoxybenzyl)-N-(alpha-methyl-p-methoxybenzyl)amide to either zeta-chloro or zeta-hydroxy substituted tert-butyl (E)-hept-2-enoate, or epsilon-chloro or epsilon-hydroxy substituted tert-butyl (E)-hex-2-enoate. Activation of the omega-substituent as a leaving group led to SN2-type ring-closure, which occurred with concomitant N-debenzylation via an E1-type deprotection step, to give the corresponding pyrrolidine or piperidine in good yield. Subsequent alkylation of these enantiopure azacycles, followed by a second ring-closure/concomitant N-debenzylation step formed the pyrrolizidine, indolizidine or quinolizidine motif, and reduction with LiAlH4 gave the target compounds in diastereoisomerically and enantiomerically pure form.
Organocatalytic asymmetric Mannich cyclization of hydroxylactams with acetals: total syntheses of (-)-epilupinine, (-)-tashiromine, and (-)-trachelanthamidine.[Pubmed:25264221]
Angew Chem Int Ed Engl. 2014 Nov 24;53(48):13196-200.
An asymmetric, organocatalytic, one-pot Mannich cyclization between a hydroxylactam and acetal is described to provide fused, bicyclic alkaloids bearing a bridgehead N atom. Both aliphatic and aromatic substrates were used in this transformation to furnish chiral pyrrolizidinone, indolizidinone, and quinolizidinone derivatives in up to 89% yield and 97% ee. The total syntheses of (-)-epiLupinine, (-)-tashiromine, and (-)-trachelanthamidine also achieved to demonstrate the generality of the process.
An efficient asymmetric synthesis of (-)-lupinine.[Pubmed:24938152]
Chem Commun (Camb). 2014 Aug 7;50(61):8309-11.
The asymmetric synthesis of (-)-Lupinine was achieved in 8 steps, 15% overall yield and >99 : 1 dr from commercially available starting materials. The strategy used for the construction of the quinolizidine scaffold involved reaction of an enantiopure tertiary dibenzylamine via two sequential ring-closures which both occurred with concomitant N-debenzylation.
Enantioselective Lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions.[Pubmed:24233720]
Science. 2013 Nov 15;342(6160):840-3.
Asymmetric catalysis of photochemical cycloadditions has been limited by the challenge of suppressing the unselective background reaction. Here, we report that the high cross-section pipi* transition of 5,6-dihydro-4-pyridones, a versatile class of enone substrates, undergoes a >50 nanometer (nm) bathochromic absorption shift upon Lewis acid coordination. Based on this observation, enantioselective intramolecular [2+2] photocycloaddition reactions (82 to 90% enantiomeric excess) were achieved with these substrates using 0.5 equivalents of a chiral Lewis acid upon irradiation at a wavelength of 366 nm. One of the products was applied as a key intermediate in the total synthesis of (+)-Lupinine and the formal synthesis of (+)-thermopsine. Several enones show similar bathochromic shifts in the presence of a Lewis acid, indicating that chiral Lewis acid catalysis may be a general approach toward enantioselective enone [2+2] photocycloadditions.
Rotational spectra of bicyclic decanes: the trans conformation of (-)-lupinine.[Pubmed:24028578]
J Phys Chem A. 2013 Dec 19;117(50):13673-9.
The conformational and structural properties of the bicyclic quinolizidine alkaloid (-)-Lupinine have been investigated in a supersonic jet expansion using microwave spectroscopy. The rotational spectrum is consistent with a single dominant trans conformation within a double-chair skeleton, which is stabilized by more than 10.4 kJ mol(-1) with respect to other conformations. In the isolated conditions of the jet, the hydroxy methyl side chain of the molecule locks in to form an intramolecular O-H...N hydrogen bond to the electron lone pair at the nitrogen atom. Accurate rotational constants, centrifugal distortion corrections, and (14)N nuclear quadrupole coupling parameters are reported and compared to ab initio (MP2) and DFT (M06-2X) calculations. The stability of Lupinine is further compared computationally with epiLupinine and decaline in order to gauge the influence of intramolecular hydrogen bonding, absent in these molecules.
Synthesis and comparison of antiplasmodial activity of (+), (-) and racemic 7-chloro-4-(N-lupinyl)aminoquinoline.[Pubmed:22901673]
Bioorg Med Chem. 2012 Oct 1;20(19):5980-5.
Recently the N-(-)-lupinyl-derivative of 7-chloro-4-aminoquinoline ((-)-AM-1; 7-chloro-4-{N-[(1S,9aR)(octahydro-2H-quinolizin-1-yl)methyl]amino}quinoline) showed potent in vitro and in vivo activity against both Chloroquine susceptible and resistant strains of Plasmodium falciparum. However, (-)-AM-1 is synthesized starting from (-)-Lupinine, an expensive alkaloid isolated from Lupinus luteus whose worldwide production is not sufficient, at present, for large market purposes. To overcome this issue, the corresponding racemic compound, derived from synthetic (+/-)-Lupinine was considered a cheaper alternative for the development of a novel antimalarial agent. Therefore, the racemic and the 7-chloro-4-(N-(+)-lupinyl)aminoquinoline ((+/-)-AM-1; (+)-AM-1) were synthesized and their in vitro antimalarial activity and cytotoxicity compared with those of (-)-AM-1. The (+)-Lupinine required for the synthesis of (+)-AM-1 was obtained through a not previously described lipase catalyzed kinetic resolution of (+/-)-Lupinine. In terms of antimalarial activity, (+/-)-AM1 and (+)-AM1 demonstrated very good activity in vitro against both CQ-R and CQ-S strains of P. falciparum (range IC(50) 16-35 nM), and low toxicity against human normal cell lines (therapeutic index >1000), comparable with that of (-)-AM1. These results confirm that the racemate (+/-)-AM1 could be considered as a potential antimalarial agent, ensuring a decrease of costs of synthesis compared to (-)-AM1.
[Isomeric derivatives of lupinine and epilupinine--organophosphorus inhibitors of cholinesterases].[Pubmed:22679755]
Ukr Biokhim Zh (1999). 2012 Jan-Feb;84(1):26-33.
The isomeric-structure analysis data of anticholinesterase action of organophosphorous inhibitors with similar structure help in the search of specific effectors and detection of differences in reactivity of various animals' enzymes. This study compared the data of efficacy in respect of 4 mammal and 5 arthropoda cholinesterase preparations for 26 quinolizidine inhibitors, which molecules contain both the isomeric unbranched and branched alkoxyl radicals in the phosphoryl group, and the epimeric Lupinine and epiLupinine derivatives in the leaving group. The changes in the alkoxyl radical structure of inhibitor molecules act on their efficacy only with respect to the mammal enzymes ("group" inhibitor specificity). The differences between Lupinine and epiLupinine derivatives were revealed. Highly specific inhibitors of different enzymes were detected among the tested compounds.
A novel sesquiterpene acid and an alkaloid from leaves of the Eastern Nigeria mistletoe, Loranthus micranthus with potent immunostimulatory activity on C57BL6 mice splenocytes and CD69 molecule.[Pubmed:21988279]
Pharm Biol. 2011 Dec;49(12):1271-6.
CONTEXT: The Eastern Nigeria mistletoe, Loranthus micranthus Linn. (Loranthaceae), is used in the treatment of several diseases including immune-modifying diseases and thus there is a need to identify the immunoactive constituents. OBJECTIVE: This research isolated and characterized the immunoactive constituents in the Eastern Nigeria mistletoe. MATERIALS AND METHODS: Bioassay-guided fractionation was employed in the isolation and purification of the constituents. The characterized compounds were screened for immunostimulatory activities on isolated C57BL/6 mice splenocytes and early activation marker, CD69 at concentrations of 10, 25, and 100 mug/mL using flow cytometry techniques and compared to lipopolysaccharide (LPS; 10 mug/mL) and concanavalin A (ConA; 2 mug/mL) as standards. RESULTS: Two compounds, a novel sesquiterpene, 2, 3-dimethoxy-benzo [a, b] cyclopentenyl-3',3',5'-trimethyl pyran-4-carboxylic acid, and a known alkaloid, Lupinine were isolated and characterized. The compounds (25 mug/mL) showed statistically significantly (p < 0.05) stimulatory activity on the splenocytes with values of 56.34 +/- 0.26% and 69.84 +/- 0.19%, respectively, compared to 7.58 +/- 0.42% recorded for the unstimulated control. Similarly, the CD69 expression assay showed immunostimulation with statistically significant values (p < 0.05) of 2.31 +/- 0.07% and 2.71 +/- 0.03%, respectively, compared to 1.69 +/- 0.05% recorded for the nonstimulated control. DISCUSSION: These data suggest that the isolated compounds possess immunomodifying abilities. In addition, the activation of the CD69 molecule is possibly one of its mechanisms of action. CONCLUSION: These compounds may be responsible in part, for the immunostimulatory activities already established for the Eastern Nigeria mistletoes.
Total syntheses of (-) epilupinine and (-)-tashiromine using imino-aldol reactions.[Pubmed:21744783]
Org Lett. 2011 Aug 5;13(15):3988-91.
Short routes to enantiomerically pure indolizidine and quinolizidine alkaloids have been developed using imino-aldol reactions of enolates derived from phenyl 5-chlorovalerate. High levels of syn selectivity (dr approximately 13-16:1) were obtained using lithium enolates of phenyl esters in combination with tert-butylsulfinyl imines. The imino-aldol adducts were deprotected and cyclized to afford (-)-epiLupinine ((-)-2) and (-)-tashiromine ((-)-1) in two further steps.


