GlucoiberinCAS# 15592-34-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

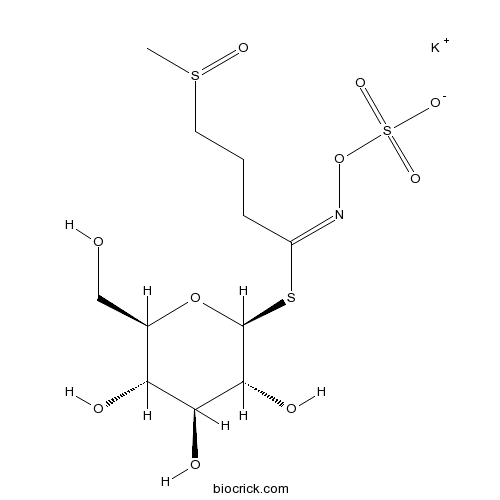
| Cas No. | 15592-34-4 | SDF | Download SDF |
| PubChem ID | 23704359 | Appearance | Powder |
| Formula | C11H20KNO10S3 | M.Wt | 461.6 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 3-(Methylsulfinyl)propylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| Chemical Name | potassium;[(E)-[4-methylsulfinyl-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylbutylidene]amino] sulfate | ||
| SMILES | CS(=O)CCCC(=NOS(=O)(=O)[O-])SC1C(C(C(C(O1)CO)O)O)O.[K+] | ||
| Standard InChIKey | PJZYNAFZGIIKQD-LUFJGRCYSA-M | ||
| Standard InChI | InChI=1S/C11H21NO10S3.K/c1-24(17)4-2-3-7(12-22-25(18,19)20)23-11-10(16)9(15)8(14)6(5-13)21-11;/h6,8-11,13-16H,2-5H2,1H3,(H,18,19,20);/q;+1/p-1/b12-7+;/t6-,8-,9+,10-,11+,24?;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glucoiberin Dilution Calculator

Glucoiberin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1664 mL | 10.8319 mL | 21.6638 mL | 43.3276 mL | 54.1594 mL |
| 5 mM | 0.4333 mL | 2.1664 mL | 4.3328 mL | 8.6655 mL | 10.8319 mL |
| 10 mM | 0.2166 mL | 1.0832 mL | 2.1664 mL | 4.3328 mL | 5.4159 mL |
| 50 mM | 0.0433 mL | 0.2166 mL | 0.4333 mL | 0.8666 mL | 1.0832 mL |
| 100 mM | 0.0217 mL | 0.1083 mL | 0.2166 mL | 0.4333 mL | 0.5416 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Glucobarbarin
Catalog No.:BCN8970
CAS No.:21087-78-5
- Phyllalbine
Catalog No.:BCN8969
CAS No.:4540-25-4
- Epiprogoitrin
Catalog No.:BCN8968
CAS No.:21087-74-1
- 4-Hydroxyglucobrassicin
Catalog No.:BCN8967
CAS No.:83327-20-2
- 4-Methoxyglucobrassicin
Catalog No.:BCN8966
CAS No.:83327-21-3
- Glucoarabin
Catalog No.:BCN8965
CAS No.:67920-64-3
- Glucocamelinin
Catalog No.:BCN8964
CAS No.:67884-10-0
- Glucoraphasatin potassium salt
Catalog No.:BCN8963
CAS No.:245550-64-5
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
A colorimetric sensor array for the discrimination of glucosinolates.[Pubmed:32480264]
Food Chem. 2020 May 26;328:127149.
A novel approach for the discrimination of different glucosinolates (sinigrin, progoitrin, gluconapin, 4-methoxyglucobrassicin, glucoraphanin, glucobrassicin, Glucoiberin, glucobrassicanapin, glucoraphenin, and glucoerucin) using a colorimetric sensor array (CSA) is reported herein. The developed CSA technique exhibited an acceptable linearity (r(2) >/= 0.97) over a concentration range of 0-150 muM for the 10 glucosinolates. The CSA coupled with principal component analysis and hierarchical cluster analysis correctly distinguished the majority of glucosinolate samples according to their type. In addition, the CSA coupled with linear discriminant analysis correctly classified the majority of 8 kinds of cruciferous vegetable samples with an overall accuracy of 94%. Furthermore, the partial least squares regression results showed that the CSA responses were correlated with the concentration in a correlation coefficient (Rp) range of 0.813-0.964. These results demonstrate that the described procedure based on the CSA technique could be useful for the rapid discrimination of different glucosinolates.
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [Glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Health-promoting phytochemicals and antioxidant capacity in different organs from six varieties of Chinese kale.[Pubmed:31889076]
Sci Rep. 2019 Dec 30;9(1):20344.
Chinese kale (Brassica oleracea var. alboglabra) has high nutritional value. This study investigated the contents of glucosinolates, antioxidants (chlorophylls, carotenoids, vitamin C, and total phenolics), and antioxidant capacity in five organs from six varieties of Chinese kale. The highest concentrations of individual and total glucosinolates were in the roots and inflorescences, respectively. The highest levels of antioxidants and antioxidant capacity were in inflorescences and leaves. Plant organs played a predominant role in glucosinolate and antioxidant accumulation. Glucoiberin, glucoraphanin, and glucobrassicin, the main anticarcinogenic glucosinolates, could be enhanced simultaneously because of their high positive correlations. The relationship between glucosinolates and antioxidant capacity indicated that glucobrassicin might contribute to the total antioxidant capacity. These results provide useful information related to consumption, breeding of functional varieties, and use of the non-edible organs of Chinese kale.
Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods.[Pubmed:31336993]
Foods. 2019 Jul 12;8(7). pii: foods8070257.
Brassica vegetables are of great interest due to their antioxidant and anti-inflammatory activity, being responsible for the glucosinolates (GLS) and their hydroxylated derivatives, the isothiocyanates (ITC). Nevertheless, these compounds are quite unstable when these vegetables are cooked. In order to study this fact, the influence of several common domestic cooking practices on the degradation of GLS and ITC in two novel Brassica spp.: broccolini (Brassica oleracea var italica Group x alboglabra Group) and kale (Brassica oleracea var. sabellica L.) was determined. On one hand, results showed that both varieties were rich in health-promoter compounds, broccolini being a good source of glucoraphanin and sulforaphane ( approximately 79 and 2.5 mg 100 g(-1) fresh weight (F.W.), respectively), and kale rich in Glucoiberin and iberin ( approximately 12 and 0.8 mg 100 g(-1) F.W., respectively). On the other hand, regarding cooking treatments, stir-frying and steaming were suitable techniques to preserve GLS and ITC (>/=50% of the uncooked samples), while boiling was deleterious for the retention of these bioactive compounds (20-40% of the uncooked samples). Accordingly, the appropriate cooking method should be considered an important factor to preserve the health-promoting effects in these trending Brassica.
Effect of Cooking Method on Antioxidant Compound Contents in Cauliflower.[Pubmed:31328127]
Prev Nutr Food Sci. 2019 Jun;24(2):210-216.
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing Glucoiberin, progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, glucobrassicin, and gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Role of Major Glucosinolates in the Defense of Kale Against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris.[Pubmed:30920356]
Phytopathology. 2019 Jul;109(7):1246-1256.
Glucosinolates (GSLs) are secondary metabolites present in Brassicaceae species implicated in their defense against plant pathogens. When a pathogen causes tissue damage, the enzyme myrosinase hydrolyzes GSLs into diverse products that exhibit antimicrobial activity against a wide range of bacteria and fungi in vitro. It was demonstrated that modulation of GSL content in vivo affects plant resistance to infection by pathogens in Arabidopsis. However, the roles of specific metabolites and how they interact with pathogens are poorly understood in Brassica crops. We previously developed a set of populations of Brassica oleracea var. acephala L. (kale) differing in content of three GSLs: the aliphatics sinigrin (2-propenyl [SIN]) and Glucoiberin (3-methylsulphinylpropyl [GIB]) and the indolic glucobrassicin (3-indolylmethyl [GBS]). These populations can be used to study the effects of major GSLs in kale, with the advantage that genotypes within each selection have the same genetic background. This research aimed to explore the role of SIN, GIB, and GBS in the defense of kale against the necrotrophic fungus Sclerotinia sclerotiorum and the bacterium Xanthomonas campestris pv. campestris. Results showed that increasing the amount of a particular GSL did not always result in disease resistance. The effects of GSLs were apparently dependent on the pathogen and the type of GSL. Thus, the aliphatic SIN was inhibitory to infection by S. sclerotiorum and the indolic GBS was inhibitory to infection by X. campestris pv. campestris. Other factors, including the quantity and proportion of other metabolites modified during the pathogen infection process, could also modulate the degree of inhibition to the pathogen.
Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria.[Pubmed:30827654]
Food Chem. 2019 Jul 15;286:616-623.
This study investigated the effect of lactic acid bacteria (LAB) fermentation on the chemical profile of autoclaved broccoli puree, using 7 broccoli-derived LAB isolates (named F1-F5, BF1 and BF2). The total concentrations of glucosinolates (Glucoiberin, progoitrin and glucoraphanin) and 10 major phenolics significantly increased from trace level and 289mug total phenolics/g dry weight (DW) respectively in autoclaved broccoli to 55 to approximately 359mug/g DW and 903 to approximately 3105mug/g DW respectively in LAB fermented broccoli puree. Differential impacts of LAB isolates on the chemical composition of autoclaved broccoli were observed, with the major differences being the significant increase in phloretic acid after fermentation by F1-F5 and an elevated glucoraphanin level in ferments by F1 and BF2. LAB fermentation is a promising way to increase the content of glucosinolates and polyphenolic compounds in broccoli, making the ferments attractive for use as functional ingredients or as a whole functional food.
Ascorbic Acid and Glucosinolate Levels in New Czech Cabbage Cultivars: Effect of Production System and Fungal Infection.[Pubmed:30046026]
Molecules. 2018 Jul 25;23(8). pii: molecules23081855.
Nutritional value and disease-preventive effects of cabbage are well-known. Levels of the antioxidant compounds ascorbic acid (AA) and glucosinolates (GSL) in new Czech cabbage cultivars were determined in the context of different production systems. The contents of AA and GSLs in cabbage biomass were determined by HPLC. Individual GSLs were identified according to their exact masses with sinigrin used as the external standard. Artificial infection with A. brassicicola generally raised the AA levels. The major GSLs (>/=10 mg kg(-1)) were glucobrassicin, sinigrin, and Glucoiberin. Indole and aliphatic GSLs were present, but no aromatic ones were detected. Ecological growth conditions and the artificial fungal infection increased the total content of GSLs and, also, of the methoxylated indole GSLs. Sulforaphane, iberin, indole-3-carbinol, and ascorbigen resulting from the hydrolysis of GSLs were found in both cultivars. The amounts and profiles of GSLs present in the two Czech cultivars demonstrated their good nutritional value. The decomposition products sulforaphane, iberin, indole-3-carbinol, and ascorbigen detected improve its health-promoting qualities and represent a suitable component of the human diet.
Formation of Sulforaphane and Iberin Products from Thai Cabbage Fermented by Myrosinase-Positive Bacteria.[Pubmed:29671807]
Molecules. 2018 Apr 19;23(4). pii: molecules23040955.
Myrosinase-positive bacteria from local fermented foods and beverages in Thailand with the capacity to metabolize glucosinolate and produce isothiocyanates (ITCs) were isolated and used as selected strains for Thai cabbage fermentation. Enterobacter xiangfangensis 4A-2A3.1 (EX) from fermented fish and Enterococcus casseliflavus SB2X2 (EC) from fermented cabbage were the two highest ITC producers among seventeen strains identified by 16S rRNA technique. EC and EX were used to ferment Thai cabbage (Brassica oleracea L. var. capitata) containing Glucoiberin, glucoraphanin and 4-hydroxyglucobrassicin at 430.5, 615.1 and 108.5 micromol/100 g DW, respectively for 3 days at 25 degrees C. Different amounts of iberin nitrile, iberin, sulforaphane and indole 3-acetonitrile were produced by spontaneous, EX- and EC-induced cabbage fermentations, and significantly higher ITCs were detected (p < 0.01) with increased antioxidant activities. Iberin and sulforaphane production in EX-induced treatment peaked on day 2 at 117.4 and 294.1 micromol/100 g DW, respectively, significantly higher than iberin at 51.7 micromol/100 g DW but not significantly higher than sulforaphane at 242.6 micromol/100 g DW in EC-induced treatment at day 2. Maximum health-promoting benefits from this functional food can be obtained from consumption of a liquid portion of the fermented cabbage with higher ITC level along with a solid portion.
Bioavailability of Isothiocyanates From Broccoli Sprouts in Protein, Lipid, and Fiber Gels.[Pubmed:29532635]
Mol Nutr Food Res. 2018 Sep;62(18):e1700837.
SCOPE: Optimization of bioavailability of dietary bioactive health-beneficial compounds is as important as increasing their concentration in foods. The aim of this study is to explore the change in bioavailability of isothiocyanates (ITCs) in broccoli sprouts incorporated in protein, fiber, and lipid gels. METHODS AND RESULTS: Five participants took part in a cross-over study and collected timed urine samples up to 24 h after consumption of proteins, dietary fibers, and lipid gels containing broccoli sprouts powder. Sulforaphane and iberin metabolites were determined in the urine samples. Samples in which sulforaphane and iberin were preformed by myrosinase led to a higher bioavailability of those compounds. Compared to the control broccoli sprout, incorporation of sprouts in gels led to lower bioavailability for preformed sulforaphane and iberin (although for sulforaphane the lower bioavailability was not significantly different) whereas for the gels rich in their precursors, glucoraphanin and Glucoiberin, the opposite trend was observed (although not significantly different). CONCLUSION: This explorative study suggests that ITCs bioavailability can be modulated by food structure and composition and further and deeper investigations are needed to develop food products that lead to an optimized ITCs bioavailability.
Rapid and Efficient Desulfonation Method for the Analysis of Glucosinolates by High-Resolution Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry.[Pubmed:29161816]
J Agric Food Chem. 2017 Dec 20;65(50):11100-11108.
The goal of our present research was to develop a simple and rapid method for the quantitation of desulfoglucosinolates (desulfoGLS) without using column chromatography. The proposed method involves extraction, concentration, incubation of glucosinolates with a sulfatase enzyme, and HPLC analysis. Identification of desulfoGLS in green kohlrabi was performed by LC-HR-ESI-QTOF-MS in positive-ionization mode. A total of 11 desulfoGLS were identified with neoglucobrassicin (3.32 +/- 0.05 mumol/g DW) as the predominant indolyl, whereas progoitrin and sinigrin were the major aliphatic desulfoGLS. The levels of the aliphatic desulfoGLS Glucoiberin, progoitrin, and glucoerucin at 7 h were found to be 3.6-, 1.9-, and 1.6-fold higher, respectively, than those produced through the conventional method. This technique was successfully applied in the identification of desulfoGLS from cabbage. The developed method has fewer unit operations, has maximum recovery, and is reproducible in the determination of desulfoGLS in a large number of Brassicaceae samples in a short time.
Reconstructing Biosynthetic Pathway of the Plant-Derived Cancer Chemopreventive-Precursor Glucoraphanin in Escherichia coli.[Pubmed:29149798]
ACS Synth Biol. 2018 Jan 19;7(1):121-131.
Epidemiological data confirmed a strong correlation between regular consumption of cruciferous vegetables and lower cancer risk. This cancer preventive property is mainly attributed to the glucosinolate products, such as glucoraphanin found in broccoli that is derived from methionine. Here we report the first successful reconstruction of the complete biosynthetic pathway of glucoraphanin from methionine in Escherichia coli via gene selection, pathway design, and protein engineering. We used branched-chain amino transferase 3 to catalyze two transamination steps to ensure the purity of precursor molecules and used cysteine as a sulfur donor to simplify the synthesis pathway. Two chimeric cytochrome P450 enzymes were engineered and expressed in E. coli functionally. The original plant C-S lyase was replaced by the Neurospora crassa hercynylcysteine sulfoxide lyase. Other pathway enzymes were successfully mined from Arabidopsis thaliana, Brassica rapa, and Brassica oleracea. Biosynthesis of glucoraphanin upon coexpression of the optimized enzymes in vivo was confirmed by liquid chromatography-tandem mass spectrometry analysis. No other glucosinolate analogues (except for Glucoiberin) were identified that could facilitate the downstream purification processes. Production of glucoraphanin in this study laid the foundation for microbial production of such health-beneficial glucosinolates in a large-scale.
Stir-Frying of Chinese Cabbage and Pakchoi Retains Health-Promoting Glucosinolates.[Pubmed:29134463]
Plant Foods Hum Nutr. 2017 Dec;72(4):439-444.
Stir-frying is a cooking method, originating from Asia, in which food is fried in small amount of very hot oil. Nowadays in many other parts of the world stir-frying is a very popular method to prepare vegetables, because it is fast and fried vegetables are tasty. However, the retention of phytochemicals like the health-beneficial glucosinolates in Brassica vegetables is less explored for stir-frying in comparison to other cooking methods. This study investigates the retention of glucosinolates in Chinese cabbage (Brassica rapa ssp. pekinensis) and pakchoi (Brassica rapa ssp. chinensis) as affected by stir-frying at various cooking durations and temperatures. Stir-frying experiments were performed at set pan temperatures ranging from 160 to 250 degrees C for a duration of 1 to 8 min. Results showed that aliphatic glucobrassicanapin is the most abundant glucosinolate identified in fresh Chinese cabbage and pakchoi, contributing for 48 and 63% of the total glucosinolate content, respectively, followed by Glucoiberin and gluconapin. Stir-frying retains the glucosinolates even at the highest temperature applied. Such retention is explained by the quick inactivation of the glucosinolate-hydrolytic enzyme myrosinase during the first minutes of frying, and by the thermal stability of the glucosinolates at those temperature/time conditions. Moreover, due to the absence of a separate water phase, leaching losses did not occur, in contrast to what is observed when boiling Brassica vegetables. These results show that stir-frying may be a suitable health-beneficial cooking option that prevents the loss of glucosinolates.
The ability to manipulate plant glucosinolates and nutrients explains the better performance of Bemisia tabaci Middle East-Asia Minor 1 than Mediterranean on cabbage plants.[Pubmed:28861220]
Ecol Evol. 2017 Jun 30;7(16):6141-6150.
The performance of herbivorous insects is greatly affected by host chemical defenses and nutritional quality. Some herbivores have developed the ability to manipulate plant defenses via signaling pathways. It is currently unclear, however, whether a herbivore can benefit by simultaneously reducing plant defenses and enhancing plant nutritional quality. Here, we show that the better performance of the whitefly Bemisia tabaci Middle East-Asia Minor 1 (MEAM1; formerly the "B" biotype) than Mediterranean (MED; formerly the "Q" biotype) on cabbage is associated with a suppression of glucosinolate (GS) content and an increase in amino acid supply in MEAM1-infested cabbage compared with MED-infested cabbage. MEAM1 had higher survival, higher fecundity, higher intrinsic rate of increase (rm), a longer life span, and a shorter developmental time than MED on cabbage plants. Amino acid content was higher in cabbage infested with MEAM1 than MED. Although infestation by either biotype decreased the levels of total GS, aliphatic GS, Glucoiberin, sinigrin, glucobrassicin, and 4OH-glucobrassicin, and the expression of related genes in cabbage, MED infestation increased the levels of 4ME-glucobrassicin, neoglucobrassicin, progoitrin, and glucoraphanin. The GS content and expression of GS-related genes were higher in cabbage infested with MED than with MEAM1. Our results suggest that MEAM1 performs better than MED on cabbage by manipulating host defenses and nutritional quality.


