GluconapinCAS# 245550-57-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

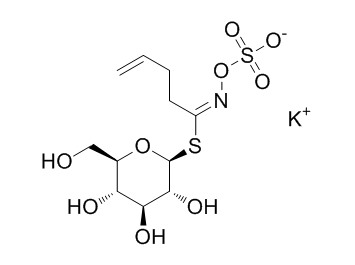
| Cas No. | 245550-57-6 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Beige powder |
| Formula | C11H18KNO9S2 | M.Wt | 411.5 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 3-Butenylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Gluconapin Dilution Calculator

Gluconapin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4301 mL | 12.1507 mL | 24.3013 mL | 48.6027 mL | 60.7533 mL |
| 5 mM | 0.486 mL | 2.4301 mL | 4.8603 mL | 9.7205 mL | 12.1507 mL |
| 10 mM | 0.243 mL | 1.2151 mL | 2.4301 mL | 4.8603 mL | 6.0753 mL |
| 50 mM | 0.0486 mL | 0.243 mL | 0.486 mL | 0.9721 mL | 1.2151 mL |
| 100 mM | 0.0243 mL | 0.1215 mL | 0.243 mL | 0.486 mL | 0.6075 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- Glucolimnanthin
Catalog No.:BCN8971
CAS No.:111810-95-8
- Progoitrin
Catalog No.:BCN8985
CAS No.:21087-77-4
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
- Achyranthoside C
Catalog No.:BCN8988
CAS No.:168009-90-3
- Damulin B
Catalog No.:BCN8989
CAS No.:1202868-75-4
- Eupalinolide O
Catalog No.:BCN8990
CAS No.:2170228-67-6
A colorimetric sensor array for the discrimination of glucosinolates.[Pubmed:32480264]
Food Chem. 2020 May 26;328:127149.
A novel approach for the discrimination of different glucosinolates (sinigrin, progoitrin, Gluconapin, 4-methoxyglucobrassicin, glucoraphanin, glucobrassicin, glucoiberin, glucobrassicanapin, glucoraphenin, and glucoerucin) using a colorimetric sensor array (CSA) is reported herein. The developed CSA technique exhibited an acceptable linearity (r(2) >/= 0.97) over a concentration range of 0-150 muM for the 10 glucosinolates. The CSA coupled with principal component analysis and hierarchical cluster analysis correctly distinguished the majority of glucosinolate samples according to their type. In addition, the CSA coupled with linear discriminant analysis correctly classified the majority of 8 kinds of cruciferous vegetable samples with an overall accuracy of 94%. Furthermore, the partial least squares regression results showed that the CSA responses were correlated with the concentration in a correlation coefficient (Rp) range of 0.813-0.964. These results demonstrate that the described procedure based on the CSA technique could be useful for the rapid discrimination of different glucosinolates.
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), progoitrin (PRO), epi-progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and Gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Differential expression of major genes involved in the biosynthesis of aliphatic glucosinolates in intergeneric Baemoochae (Brassicaceae) and its parents during development.[Pubmed:31792713]
Plant Mol Biol. 2020 Jan;102(1-2):171-184.
KEY MESSAGE: Thus study found the temporal and spatial relationship between production of aliphatic glucosinolate compounds and the expression profile of glucosinolate-related genes during growth and development in radish, Chinese cabbage, and their intergeneric hybrid baemoochae plants. Glucosinolates (GSLs) are one of major bioactive compounds in Brassicaceae plants. GSLs play a role in defense against microbes as well as chemo-preventative activity against cancer, which draw attentions from plant scientists. We investigated the temporal relationship between production of aliphatic Glucosinolate (GSLs) compounds and the expression profile of GSL related genes during growth and development in radish, Chinese cabbage, and their intergeneric hybrid, baemoochae. Over the complete life cycle, Glucoraphasatin (GRH) and glucoraphanin (GRE) predominated in radish, whereas Gluconapin (GNP), glucobrassicanapin (GBN), and glucoraphanin (GRA) abounded in Chinese cabbage. Baemoochae contained intermediate levels of all GSLs studied, indicating inheritance from both radish and Chinese cabbage. Expression patterns of BCAT4, CYP79F1, CYP83A1, UGT74B1, GRS1, FMOgs-ox1, and AOP2 genes showed a correlation to their corresponding encoded proteins in radish, Chinese cabbage, and baemoochae. Interestingly, there is a sharp change in gene expression pattern involved in side chain modification, particularly GRS1, FMOgs-ox1, and AOP2, among these plants during the vegetative and reproductive stage. For instance, the GRS1 was strongly expressed during leaf development, while both of FMOgs-ox1 and AOP2 was manifested high in floral tissues. Furthermore, expression of GRS1 gene which is responsible for GRH production was predominantly expressed in leaf tissues of radish and baemoochae, whereas it was only slightly detected in Chinese cabbage root tissue, explaining why radish has an abundance of GRH compared to other Brassica plants. Altogether, our comprehensive and comparative data proved that aliphatic GSLs biosynthesis is dynamically and precisely regulated in a tissue- and development-dependent manner in Brassicaceae family members.
The optimal mixing ratio of Brassica napus and Brassica juncea meal improve nematode Meloidogyne hapla effects.[Pubmed:31610733]
Plant Signal Behav. 2019;14(12):1678369.
The use of rapeseed (Brassica napus L.) or leaf mustard (Brassica juncea L. Czern) meal or both as organic fertilizer not only improves the soil environment and crop productivity by supplying nutrients but also has nematicidal effects. This study aimed to establish the optimal application levels of rapeseed and leaf mustard meal for stronger nematode control in tomato. Tomato is one of the most important solanaceous crops which is severely damaged by nematodes. At first, meal (120 g of varying mixing ratios of rapeseed and leaf mustard meal) was mixed with sterilized soil (1 kg). The optimal ratio of rapeseed:leaf mustard meal for effective nematode control was 20:100 g/kg of soil. Progoitrin and Gluconapin were the most abundant glucosinolates found in rapeseed meal, while sinigrin was the most abundant in leaf mustard meal. The amount of sinigrin increased if the leaf mustard meal proportion increased in the meal mixture. Although the content of sinigrin in optimal ratio mixture of rapeseed and leaf mustard meal is lower than only leaf mustard meal, it is presumed that nematocidal effects of the mixture are better than that of the single component due to the high contents of progoitrin and Gluconapin. So, we propose that rapeseed and leaf mustard meal mixture at an appropriate ratio can be used as an environmentally friendly nematocide.
Assessing the Fate and Bioavailability of Glucosinolates in Kale (Brassica oleracea) Using Simulated Human Digestion and Caco-2 Cell Uptake Models.[Pubmed:31374175]
J Agric Food Chem. 2019 Aug 28;67(34):9492-9500.
Glucosinolates and their hydrolysis products were characterized in fresh and in in vitro gastric and intestinal digesta of Dinosaur kale (Brassica oleracea L var. palmifolia DC). In fresh kale, glucoraphanin, sinigrin, Gluconapin, gluconasturtiin, glucoerucin, glucobrasscin, and 4-methoxylglucobrassicin were identified. After 120 min of gastric digestion, the levels of glucoraphanin, sinigrin, and Gluconapin decreased, and no glucoerucin or glucobrasscin was detected. However, a concomitant increase in the glucosinolate hydrolysis products allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane was observed. This trend continued through intestinal digestion. After 120 min, the levels of allyl nitrile, 3-butenyl isothiocyanate, phenylacetonitrile, and sulforaphane were 88.19 +/- 5.85, 222.15 +/- 30.26, 129.17 +/- 17.57, and 13.71 +/- 0.62 pmol/g fresh weight, respectively. Intestinal digesta were then applied to Caco-2 cell monolayers to assess the bioavailability. After 6 h of incubation, no glucosinolates were detected and the percentage of total cellular uptake of the glucosinolate hydrolysis products ranged from 29.35% (sulforaphane) to 46.60% (allyl nitrile).
Effect of Cooking Method on Antioxidant Compound Contents in Cauliflower.[Pubmed:31328127]
Prev Nutr Food Sci. 2019 Jun;24(2):210-216.
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing glucoiberin, progoitrin, glucoraphanin, sinigrin, Gluconapin, glucoiberverin, glucobrassicin, and gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Overexpression of the MYB29 transcription factor affects aliphatic glucosinolate synthesis in Brassica oleracea.[Pubmed:31190320]
Plant Mol Biol. 2019 Sep;101(1-2):65-79.
KEY MESSAGE: Overexpression of BoMYB29 gene up-regulates the aliphatic glucosinolate pathway in Brassica oleracea plants increasing the production of the anti-cancer metabolite glucoraphanin, and the toxic and pungent sinigrin. Isothiocyanates, the bio-active hydrolysis products of glucosinolates, naturally produced by several Brassicaceae species, play an important role in human health and agriculture. This study aims at correlating the content of aliphatic glucosinolates to the expression of genes involved in their synthesis in Brassica oleracea, and perform functional analysis of BoMYB29 gene. To this purpose, three genotypes were used: a sprouting broccoli, a cabbage, and a wild genotype (Winspit), a high glucosinolate containing accession. Winspit showed the highest transcript level of BoMYB28, BoMYB29 and BoAOP2 genes, and BoAOP2 expression was positively correlated with that of the two MYB genes. Further analyses of the aliphatic glucosinolates also showed a positive correlation between the expression of BoAOP2 and the production of sinigrin and Gluconapin in Winspit. The Winspit BoMYB29 CDS was cloned and overexpressed in Winspit and in the DH AG1012 line. Overexpressing Winspit plants produced higher quantities of alkenyl glucosinolates, such as sinigrin. Conversely, the DH AG1012 transformants showed a higher production of methylsulphinylalkyl glucosinolates, including glucoraphanin, and, despite an up-regulation of the aliphatic glucosinolate genes, no increase in alkenyl glucosinolates. The latter may be explained by the absence of a functional AOP2 gene in DH AG1012. Nevertheless, an extract of DH AG1012 lines overexpressing BoMYB29 provided a chemoprotective effect on human colon cells. This work exemplifies how the genetic diversity of B. oleracea may be used by breeders to select for higher expression of transcription factors for glucosinolate biosynthesis to improve its natural, health-promoting properties.
Bunias erucago L.: Glucosinolate Profile and In Vitro Biological Potential.[Pubmed:30791395]
Molecules. 2019 Feb 19;24(4). pii: molecules24040741.
Bunias erucago belongs to the Brassicaceae family, which represents a forgotten crop of the Euro-Mediterranean area. The aim of the present study was to determine the glucosinolate profile in different plant parts and biological properties (antioxidant, anticholinesterase, and cytotoxic activities) of the isolates containing glucosinolate breakdown products. The chemical profiles were determined by using HPLC-PDA-MS/MS of desulfoglucosinolates and GC-MS of glucosinolate degradation products. The analysis of B. erucago showed the presence of seven glucosinolates: Gluconapin (1), glucoraphasatin (2), glucoraphenin (3), glucoerucin (4), glucoraphanin (5), glucotropaeolin (6), and glucosinalbin (7). The total glucosinolate content ranged from 7.0 to 14.6 micromol/g of dry weight, with the major glucosinolate glucosinalbin in all parts. The antioxidant activity of all volatile isolates was not notable. At a tested concentration of 227 mug/mL, flower hydro-distillate (FH) showed good AChE inhibition, i.e., 40.9%, while root hydro-distillate (RH) had good activity against BChE, i.e., 54.3%. FH showed the best activity against both tested human bladder cancer cell lines, i.e., against T24 after 72 h, which have IC50 of 16.0 mug/mL, and against TCCSUP after 48 h with IC50 of 7.8 mug/mL, and can be considered as highly active. On the other hand, RH showed weak activity against tested cancer cells.
Variation of glucosinolates on position orders of flower buds in turnip rape (Brassica rapa).[Pubmed:30174493]
Saudi J Biol Sci. 2017 Nov;24(7):1562-1566.
To glucosinolate (GSL) contents on flower buds depending on their position orders in turnip rape (Brassica rapa), three Japanese 'Nabana' cultivars such as cv. No. 21 (Brassica rapa, early type), cv. Husanohana (B. rapa, late type) and cv. Norin No. 20 (B. napus) were investigated using HPLC analysis. Ten GSLs including glucoraphanin, sinigrin, glucoalyssin, napoleiferin, Gluconapin, 4-hydroxyglucobrassicin, glucobrassicanapin, glucobrassicin, and gluconasturtiin were detected. Differences in individual and total GSL contents were found between two plant varieties, which are also depending on various developmental stages. Among the GSLs, Gluconapin (mean 23.11 mumole/g dry weight (DW) and glucobrassicanapin (mean 13.41 mumole/g DW) documented the most abundant compounds and contributed average 39 and 27% of the total GSLs, but indolyl and aromatic GSLs together accounted >10% of the total GSLs. The presence of significant quantities of Gluconapin in the cultivars should be studied more extensively, since the GSL is mainly responsible for the bitter taste.
Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa.[Pubmed:30056602]
Bioprocess Biosyst Eng. 2018 Nov;41(11):1665-1677.
Glucosinolates (GSLs) and phenolic compounds (PCs) are biologically active and involved in the defense reaction of plants; these compounds have a beneficial effect on human health. In this study, we described the influence of biologically synthesized silver nanoparticles (Ag NPs) to enhance the phytochemicals (GSLs and PCs), their transcription levels, and their biological activities in genetically transformed root cultures (hairy root cultures) of Brassica rapa. The concentrations of silver and reactive oxygen species (malondialdehyde and hydrogen peroxide) were highly elevated in the Ag NP-elicited hairy roots (HRs). Glucosinolates (glucoallysin, glucobrassicanapin, sinigrin, progoitrin, Gluconapin, 4-methoxyglucobrassicin, 4-hydroxyglucobrassicin, glucobrassicin, neoglucobrassicin, and gluconasturtiin) and their transcripts (MYB34, MYB51, MYB28, and MYB29) were significantly enhanced in the Ag NP-elicited HRs. Moreover, the phenolic compounds (flavonols, hydroxybenzoic, and hydroxycinnamic acids) were significantly enriched in the Ag NP-elicited HRs. Total phenolic and flavonoid concentrations and their transcripts (PAL, CHI, and FLS) were higher in the Ag NP-elicited HRs than in the non-elicited HRs. Additionally, biological (antioxidant, antimicrobial, and anticancer) activities were significantly higher in the Ag NP-elicited HRs than in the non-elicited HRs. The Ag NP-elicited HR cultures offered an efficient and promising in vitro method to increase the production of health-promoting bioactive compounds, which may be useful in nutraceutical and pharmaceutical industries.
Jasmonic Acid-Mediated Aliphatic Glucosinolate Metabolism Is Involved in Clubroot Disease Development in Brassica napus L.[Pubmed:29922320]
Front Plant Sci. 2018 Jun 4;9:750.
Glucosinolate (GSL) is associated with clubroot disease, which is caused by the obligate biotrophic protist Plasmodiophora brassicae. Due to the complicated composition of GSLs, their exact role in clubroot disease development remains unclear. By investigating clubroot disease resistance in cruciferous plants and characterizing the GSL content in seeds, we can determine if clubroot disease development is related to the components of GSLs. The difference in the infection process between Matthiola incana L. (resistant) and Brassica napus L. (susceptible) was determined. Root hair infection was definitely observed in both resistant and susceptible hosts, but no infection was observed during the cortical infection stage in resistant roots; this finding was verified by molecular detection of P. brassicae via PCR amplification at various times after inoculation. Based on the time course detection of the contents and compositions of GSLs after P. brassicae inoculation, susceptible roots exhibited increased accumulation of aliphatic, indolic, and aromatic GSLs in B. napus, but only aromatic GSLs were significantly increased in M. incana. Gluconapin, which was the main aliphatic GSL in B. napus and present only in B. napus, was significantly increased during the secondary infection stage. Quantification of the internal jasmonic acid (JA) concentration showed that both resistant and susceptible plants exhibited an enhanced level of JA, particularly in susceptible roots. The exogenous JA treatment induced aliphatic GSLs in B. napus and aromatic GSLs in M. incana. JA-induced aromatic GSLs may be involved in the defense against P. brassicae, whereas aliphatic GSLs induced by JA in B. napus likely play a role during the secondary infection stage. Three candidate MYB28 genes regulate the content of aliphatic GSLs identified in B. napus; one such gene was BnMYB28.1, which was significantly increased following both the treatment with exogenous JA and P. brassicae inoculation. In summary, the increased content of JA during the secondary infection stage may induce the expression of BnMYB28.1, which caused the accumulation of aliphatic GSLs in clubroot disease development.
Identification of redox imbalance as a prominent metabolic response elicited by rapeseed feeding in swine metabolome.[Pubmed:29518202]
J Anim Sci. 2018 May 4;96(5):1757-1768.
Rapeseed (RS) is an abundant and inexpensive source of energy and AA in diets for monogastrics and a sustainable alternative to soybean meal. It also contains diverse bioactive phytochemicals that could have antinutritional effects at high dose. When the RS-derived feed ingredients (RSF) are used in swine diets, the uptake of these nutrients and phytochemicals is expected to affect the metabolic system. In this study, 2 groups of young pigs (17.8 +/- 2.7 kg initial BW) were equally fed a soybean meal-based control diet and an RSF-based diet, respectively, for 3 wk. Digesta, liver, and serum samples from these pigs were examined by liquid chromatography-mass spectrometry-based metabolomic analysis to determine the metabolic effects of the 2 diets. Analyses of digesta samples revealed that sinapine, sinapic acid, and Gluconapin were robust exposure markers of RS. The distribution of free AA along the intestine of RSF pigs was consistent with the reduced apparent ileal digestibility of AA observed in these pigs. Despite its higher fiber content, the RSF diet did not affect microbial metabolites in the digesta, including short-chain fatty acids and secondary bile acids. Analyses of the liver and serum samples revealed that RSF altered the levels of AA metabolites involved in the urea cycle and 1-carbon metabolism. More importantly, RSF increased the levels of multiple oxidized metabolites and aldehydes while decreased the levels of ascorbic acid and docosahexaenoic acid-containing lipids in the liver and serum, suggesting that RSF could disrupt redox balance in young pigs. Overall, the results indicated that RSF elicited diverse metabolic events in young pigs through its influences on nutrient and antioxidant metabolism, which might affect the performance and health in long-term feeding and also provide the venues for nutritional and processing interventions to improve the utilization of RSF in pigs.


