ProgoitrinCAS# 21087-77-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 21087-77-4 | SDF | Download SDF |
| PubChem ID | 138375859 | Appearance | Beige powder |
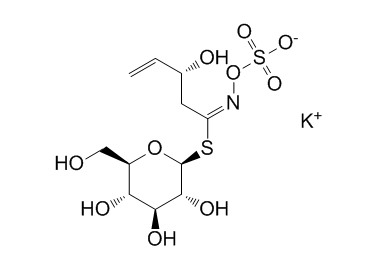
| Formula | C11H18KNO10S2 | M.Wt | 427.5 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Glucorapiferin potassium salt; 2(R)-Hydroxy 3-butenylglucosinolate potassium salt | ||
| Solubility | Soluble in methanol and water | ||
| Chemical Name | potassium;[(E)-[(3R)-3-hydroxy-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanylpent-4-enylidene]amino] sulfate | ||
| SMILES | C=CC(CC(=NOS(=O)(=O)[O-])SC1C(C(C(C(O1)CO)O)O)O)O.[K+] | ||
| Standard InChIKey | GAYXFCKWHSTEIK-KRGOUHSNSA-M | ||
| Standard InChI | InChI=1S/C11H19NO10S2.K/c1-2-5(14)3-7(12-22-24(18,19)20)23-11-10(17)9(16)8(15)6(4-13)21-11;/h2,5-6,8-11,13-17H,1,3-4H2,(H,18,19,20);/q;+1/p-1/b12-7+;/t5-,6+,8+,9-,10+,11-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Progoitrin Dilution Calculator

Progoitrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3392 mL | 11.6959 mL | 23.3918 mL | 46.7836 mL | 58.4795 mL |
| 5 mM | 0.4678 mL | 2.3392 mL | 4.6784 mL | 9.3567 mL | 11.6959 mL |
| 10 mM | 0.2339 mL | 1.1696 mL | 2.3392 mL | 4.6784 mL | 5.848 mL |
| 50 mM | 0.0468 mL | 0.2339 mL | 0.4678 mL | 0.9357 mL | 1.1696 mL |
| 100 mM | 0.0234 mL | 0.117 mL | 0.2339 mL | 0.4678 mL | 0.5848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gluconapin
Catalog No.:BCN8984
CAS No.:245550-57-6
- Neoglucobrassicin potassium salt
Catalog No.:BCN8983
CAS No.:5187-84-8
- Glucoalyssin
Catalog No.:BCN8982
CAS No.:499-37-6
- Lupinine
Catalog No.:BCN8981
CAS No.:486-70-4
- Glucomoringin
Catalog No.:BCN8979
CAS No.:316165-49-8
- Glucotropaeolin
Catalog No.:BCN8978
CAS No.:5115-71-9
- Glucoerucin
Catalog No.:BCN8977
CAS No.:15592-37-7
- Glucoberteroin
Catalog No.:BCN8976
CAS No.:245550-65-6
- Glucoiberin
Catalog No.:BCN8975
CAS No.:15592-34-4
- Glucobrassicanapin
Catalog No.:BCN8974
CAS No.:245550-58-7
- Sinalbin
Catalog No.:BCN8973
CAS No.:20196-67-2
- Glucocapparin
Catalog No.:BCN8972
CAS No.:15592-33-3
- 18-Hydroxyspartioidine
Catalog No.:BCN8941
CAS No.:
- Heliotridine N-oxide
Catalog No.:BCN8944
CAS No.:
- Rinderine hydrochloride
Catalog No.:BCN8947
CAS No.:26131-12-4
- Jacoline N-oxide
Catalog No.:BCN8954
CAS No.:
- Merepoxine N-oxide
Catalog No.:BCN8956
CAS No.:
- 11-(Methylsulfinyl)undecylglucosinolate
Catalog No.:BCN8980
CAS No.:
- Pongamol
Catalog No.:BCN8986
CAS No.:484-33-3
- Hispidulin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN8987
CAS No.:244285-12-9
- Achyranthoside C
Catalog No.:BCN8988
CAS No.:168009-90-3
- Damulin B
Catalog No.:BCN8989
CAS No.:1202868-75-4
- Eupalinolide O
Catalog No.:BCN8990
CAS No.:2170228-67-6
- Sibiricose A1
Catalog No.:BCN8991
CAS No.:139726-40-2
A colorimetric sensor array for the discrimination of glucosinolates.[Pubmed:32480264]
Food Chem. 2020 May 26;328:127149.
A novel approach for the discrimination of different glucosinolates (sinigrin, Progoitrin, gluconapin, 4-methoxyglucobrassicin, glucoraphanin, glucobrassicin, glucoiberin, glucobrassicanapin, glucoraphenin, and glucoerucin) using a colorimetric sensor array (CSA) is reported herein. The developed CSA technique exhibited an acceptable linearity (r(2) >/= 0.97) over a concentration range of 0-150 muM for the 10 glucosinolates. The CSA coupled with principal component analysis and hierarchical cluster analysis correctly distinguished the majority of glucosinolate samples according to their type. In addition, the CSA coupled with linear discriminant analysis correctly classified the majority of 8 kinds of cruciferous vegetable samples with an overall accuracy of 94%. Furthermore, the partial least squares regression results showed that the CSA responses were correlated with the concentration in a correlation coefficient (Rp) range of 0.813-0.964. These results demonstrate that the described procedure based on the CSA technique could be useful for the rapid discrimination of different glucosinolates.
Stereoselective pharmacokinetic study of epiprogoitrin and progoitrin in rats with UHPLC-MS/MS method.[Pubmed:32416341]
J Pharm Biomed Anal. 2020 May 7;187:113356.
An accurate and precise liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method was developed and validated for the pharmacokinetic study of epiProgoitrin and Progoitrin, a pair of epimers that can be deglycosylated to epigoitrin and goitrin, respectively. These analytes were administered intravenously or intragastrically to male Sprague-Dawley rats, and the influence of 3(R/S)-configuration on the pharmacokinetics of both epimers in rat plasma was elucidated. The analytes and an internal standard (i.e., sinigrin) were resolved by LC-MS/MS on a reverse-phase ACQUITY UPLC HSS T3 column equilibrated and eluted with acetonitrile and water (0.1 % formic acid) at a fl ow rate of 0.3mL/min. Quantitation was achieved by applying the multiple reaction monitoring mode, in the negative ion mode, at transitions of m/z 388 --> 97 and m/z 358 --> 97 for the epimers and sinigrin, respectively. The method demonstrated good linearity over the concentration range of 2-5000ng/mL (r > 0.996). The lower limit of quantification for epiProgoitrin and Progoitrin was 2ng/mL. The interday and intraday accuracy and precision were within +/-15 %. The extraction recovery, stability, and matrix effect were demonstrated to be within acceptable limits. The validated method was thus successfully applied for the pharmacokinetic study of both the epimers. After the rats received the same oral dose of the epimers, the pharmacokinetic profiles were similar. The maximum plasma concentration (Cmax) and AUC values of epiProgoitrin were a bit higher than those of Progoitrin, whereas the pharmacokinetic behaviours of the epimers were obviously different upon intravenous administration. The Cmax and AUC values of epiProgoitrin were approximately three-fold higher than those of Progoitrin, and the half-life of Progoitrin was much shorter than that of epiProgoitrin. The oral bioavailability of Progoitrin was 20.1 %-34.1 %, which is three times higher than that of epiProgoitrin.
Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea var. capitata) Germplasm.[Pubmed:32316621]
Molecules. 2020 Apr 17;25(8). pii: molecules25081860.
Individual glucosinolates (GSLs) were assessed to select cabbage genotypes for a potential breeding program. One hundred forty-six cabbage genotypes from different origins were grown in an open field from March to June 2019; the cabbage heads were used for GSL analyses. Seven aliphatics [glucoiberin (GIB), Progoitrin (PRO), epi-Progoitrin (EPI), sinigrin (SIN), glucoraphanin (GRA), glucoerucin (GER) and gluconapin (GNA)], one aromatic [gluconasturtiin (GNS)] and four indolyl GSLs [glucobrassicin (GBS), 4-hydroxyglucobrassicin (4HGBS), 4-methoxyglucobrassicin (4MGBS), neoglucobrassicin (NGBS)] were found this study. Significant variation was observed in the individual GSL content and in each class of GSLs among the cabbage genotypes. Aliphatic GSLs were predominant (58.5%) among the total GSLs, followed by indolyl GSL (40.7%) and aromatic GSLs (0.8%), showing 46.4, 51.2 and 137.8% coefficients of variation, respectively. GIB, GBS and NGBS were the most common GSLs found in all genotypes. GBS was the most dominant GSL, with an average value of 3.91 micromol g(-1) (0.79 to 13.14 micromol g(-1)). SIN, GIB, PRO and GRA were the other major GSLs, showing average values of 3.45, 1.50, 0.77 and 0.62 micromol g(-1), respectively. The genotypes with relatively high contents of GBS, SIN, GIB and GRA warrant detailed studies for future breeding programs since the hydrolysis products of these GSLs have several anti-cancer properties.
Antiviral activity of Isatidis Radix derived glucosinolate isomers and their breakdown products against influenza A in vitro/ovo and mechanism of action.[Pubmed:31918015]
J Ethnopharmacol. 2020 Apr 6;251:112550.
ETHNOPHARMACOLOGICAL RELEVANCE: Isatidis Radix, the sun-dried roots of Isatis indigotica Fortune ex Lindl., is one of the most usually used traditional Chinese medicines. For centuries, the herb has been employed in clinical practice for treatment of virus infection and inflammation. However, its active ingredients remain unclear. AIM OF THE STUDY: In the present study, the anti-influenza virus activity of epiProgoitrin, Progoitrin, epigoitrin and goitrin, the Isatidis Radix derived glucosinolate isomers and their breakdown products, was firstly evaluated in vitro and in ovo and their mechanism of action was investigated. MATERIALS AND METHODS: EpiProgoitrin, Progoitrin, epigoitrin and goitrin were isolated from Isatidis Radix by chiral separation. In vitro and in ovo evaluations were performed on Madin-Darby canine kidney (MDCK) cells and embryonated eggs respectively, both using protocols including prevention, treatment and virus neutralization. Hemagglutination (HA) and neuraminidase (NA) inhibition assays were performed for further understanding of the antiviral mechanism. RESULTS: Isatidis Radix derived glucosinolate isomers and their breakdown products all exhibited dose-dependent inhibition effect against influenza A virus (H1N1) without toxicity. The antiviral potency of the components was in the order of Progoitrin > goitrin > epigoitrin > epiProgoitrin. The attachment of the constituents to the viral envelope conduced to the mechanism of their antiviral action without disturbing viral adsorption or budding. CONCLUSION: Taken together, these results are promising for further development of Isatidis Radix and may contribute an adjunct to pharmacotherapy for influenza virus infection.
Natural Variation of Glucosinolates and Their Breakdown Products in Broccoli (Brassica oleracea var. italica) Seeds.[Pubmed:31631662]
J Agric Food Chem. 2019 Nov 13;67(45):12528-12537.
Seeds of 32 pure lines and 6 commercial broccoli cultivars were used to investigate variation in glucosinolates and their breakdown products. The aliphatic glucosinolate content was 54.5-218.7 mumol/g fresh weight, accounting for >90% of the total glucosinolates. The major glucosinolates found were glucoraphanin and glucoerucin in 27 samples and Progoitrin in 7 samples. A gas chromatography-flame ionization detector (GC-FID) method was used to identify glucosinolate breakdown products; nine products were directly determined using standards. Using Arabidopsis thaliana lines myb28myb29 and Landsberg erecta to hydrolyze each reference glucosinolate, seven products were tentatively identified. 4-(Methylsulfinyl)butyl isothiocyanate and 5-(methylsulfinyl)pentanenitrile contents were 2.6-91.1 mumol/g fresh weight and 0-35.4 mumol/g fresh weight, respectively, with epithionitriles being more common than nitriles in accessions rich in alkenyl glucosinolate. Additionally, (S)-5-vinyl-1,3-oxazolidine-2-thione was detected in accessions rich in Progoitrin. Specific lines with altered glucosinolate profiles and breakdown products were obtained and discussed according to the putative glucosinolate metabolism pathway.
The optimal mixing ratio of Brassica napus and Brassica juncea meal improve nematode Meloidogyne hapla effects.[Pubmed:31610733]
Plant Signal Behav. 2019;14(12):1678369.
The use of rapeseed (Brassica napus L.) or leaf mustard (Brassica juncea L. Czern) meal or both as organic fertilizer not only improves the soil environment and crop productivity by supplying nutrients but also has nematicidal effects. This study aimed to establish the optimal application levels of rapeseed and leaf mustard meal for stronger nematode control in tomato. Tomato is one of the most important solanaceous crops which is severely damaged by nematodes. At first, meal (120 g of varying mixing ratios of rapeseed and leaf mustard meal) was mixed with sterilized soil (1 kg). The optimal ratio of rapeseed:leaf mustard meal for effective nematode control was 20:100 g/kg of soil. Progoitrin and gluconapin were the most abundant glucosinolates found in rapeseed meal, while sinigrin was the most abundant in leaf mustard meal. The amount of sinigrin increased if the leaf mustard meal proportion increased in the meal mixture. Although the content of sinigrin in optimal ratio mixture of rapeseed and leaf mustard meal is lower than only leaf mustard meal, it is presumed that nematocidal effects of the mixture are better than that of the single component due to the high contents of Progoitrin and gluconapin. So, we propose that rapeseed and leaf mustard meal mixture at an appropriate ratio can be used as an environmentally friendly nematocide.
Effect of Cooking Method on Antioxidant Compound Contents in Cauliflower.[Pubmed:31328127]
Prev Nutr Food Sci. 2019 Jun;24(2):210-216.
In this study, we determined the contents of glucosinolate, polyphenol, and flavonoid, and the antioxidant activities of uncooked, steamed, and boiled cauliflower. Eight glucosinolate peaks were detected, representing glucoiberin, Progoitrin, glucoraphanin, sinigrin, gluconapin, glucoiberverin, glucobrassicin, and gluconasturtiin. Boiled cauliflower contained significantly lowered concentrations of glucosinolate, total polyphenol, and total flavonoid compared to uncooked or steamed cauliflower. These results clearly indicate that health-promoting compounds in cauliflower are significantly impacted by different cooking methods: uncooked> steamed> boiled. The amounts of total polyphenols and total flavonoids in uncooked cauliflower extracted with 80% ethanol were higher than extracts of steamed and boiled cauliflower. The highest antioxidant activity was observed in uncooked cauliflower extracted using 80% ethanol compared to those extracted with water at the same concentration. Steamed and boiled cauliflower extracts also showed lower antioxidant activity than uncooked extracts. Based on these results, fresh uncooked cauliflower contains higher contents of health-promoting compounds and elevated antioxidant activity. Moreover, steaming may be more desirable than boiling in order to minimize loss of glucosinolates when storing, pretreating, processing, and cooking cruciferous vegetables.
Glucosinolate Content and Sensory Evaluation of Baby Leaf Rapeseed from Annual and Biennial White- and Yellow-Flowering Cultivars with Repeated Harvesting in Two Seasons.[Pubmed:31237979]
J Food Sci. 2019 Jul;84(7):1888-1899.
The chemical and sensory quality of field-grown vegetables may be influenced by cultivar choice and agronomic factors but knowledge is lacking on the new rapeseed vegetables. White- and yellow-flowering rapeseed cultivars were tested in two seasonally different field studies in Denmark at three different growing stages by early sowing the first year and late sowing the second year. Content of glucosinolates (GLSs) was analyzed, and the sensory quality of baby leaf samples was evaluated. The GLS content differed among cultivars across years in all growing stages, with biennial cultivars having the highest GLS content. In the second year, a higher content of all identified GLSs was found at two growing stages except for neoglucobrassicin and gluconasturtiin, compared to the first year. On the contrary, higher contents of all identified GLSs were found at a third stage in the first year except for Progoitrin and 4-methoxy glucobrassicin. Sensory evaluation of bitterness revealed differences among cultivars, higher intensities of bitterness in biennial cultivars, and a relationship between bitterness and content of bitter-tasting and total GLSs. The effect of repeated harvesting on GLS content differed between the years and no general pattern was seen, except that the composition of individual GLSs was comparable for the biennial cultivars. We conclude that growing season and life cycle had a stronger influence on GLS content than stage at harvest. The link between bitter-tasting GLSs and bitterness revealed that life cycle and seasonal effects affected the sensory profile of baby leaf rapeseed thereby making a healthier product due to high content of health-beneficial GLSs.
Non-enzymatic transformations of dietary 2-hydroxyalkenyl and aromatic glucosinolates in the stomach of monogastrics.[Pubmed:31006474]
Food Chem. 2019 Sep 1;291:77-86.
Monogastric animals exhibit different biological responses to structurally diverse glucosinolates and their transformation products, depending on the dietary levels. The transformations of 2-hydroxyalkenyl and aromatic glucosinolates were examined in vitro under gastric conditions, ex vivo in ligated porcine stomachs and in vivo in a rat model. Intact glucosinolates were almost completely transformed in vitro within 1h at pH 3 (73-88%) and at pH 5 (97-100%) upon addition of Fe(2+) ranging from two-fold molar excess. Glucosinolate transformations reached 78-99% when incubated ex vivo in ligated porcine stomachs. Rat in vivo feeding trials showed major reductions (81-84%) in the intact glucosinolate contents upon passage through the gastrointestinal (GI) tract. Non-enzymatic transformations of glucosinolates occur in the stomach, where pH and the level of Fe(2+) are primary determinants. This is the first study to show a complex formation between iron-Progoitrin and iron-sinalbin, facilitating the transformation into nitriles and thionamides.
Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria.[Pubmed:30827654]
Food Chem. 2019 Jul 15;286:616-623.
This study investigated the effect of lactic acid bacteria (LAB) fermentation on the chemical profile of autoclaved broccoli puree, using 7 broccoli-derived LAB isolates (named F1-F5, BF1 and BF2). The total concentrations of glucosinolates (glucoiberin, Progoitrin and glucoraphanin) and 10 major phenolics significantly increased from trace level and 289mug total phenolics/g dry weight (DW) respectively in autoclaved broccoli to 55 to approximately 359mug/g DW and 903 to approximately 3105mug/g DW respectively in LAB fermented broccoli puree. Differential impacts of LAB isolates on the chemical composition of autoclaved broccoli were observed, with the major differences being the significant increase in phloretic acid after fermentation by F1-F5 and an elevated glucoraphanin level in ferments by F1 and BF2. LAB fermentation is a promising way to increase the content of glucosinolates and polyphenolic compounds in broccoli, making the ferments attractive for use as functional ingredients or as a whole functional food.
Influence of silver nanoparticles on the enhancement and transcriptional changes of glucosinolates and phenolic compounds in genetically transformed root cultures of Brassica rapa ssp. rapa.[Pubmed:30056602]
Bioprocess Biosyst Eng. 2018 Nov;41(11):1665-1677.
Glucosinolates (GSLs) and phenolic compounds (PCs) are biologically active and involved in the defense reaction of plants; these compounds have a beneficial effect on human health. In this study, we described the influence of biologically synthesized silver nanoparticles (Ag NPs) to enhance the phytochemicals (GSLs and PCs), their transcription levels, and their biological activities in genetically transformed root cultures (hairy root cultures) of Brassica rapa. The concentrations of silver and reactive oxygen species (malondialdehyde and hydrogen peroxide) were highly elevated in the Ag NP-elicited hairy roots (HRs). Glucosinolates (glucoallysin, glucobrassicanapin, sinigrin, Progoitrin, gluconapin, 4-methoxyglucobrassicin, 4-hydroxyglucobrassicin, glucobrassicin, neoglucobrassicin, and gluconasturtiin) and their transcripts (MYB34, MYB51, MYB28, and MYB29) were significantly enhanced in the Ag NP-elicited HRs. Moreover, the phenolic compounds (flavonols, hydroxybenzoic, and hydroxycinnamic acids) were significantly enriched in the Ag NP-elicited HRs. Total phenolic and flavonoid concentrations and their transcripts (PAL, CHI, and FLS) were higher in the Ag NP-elicited HRs than in the non-elicited HRs. Additionally, biological (antioxidant, antimicrobial, and anticancer) activities were significantly higher in the Ag NP-elicited HRs than in the non-elicited HRs. The Ag NP-elicited HR cultures offered an efficient and promising in vitro method to increase the production of health-promoting bioactive compounds, which may be useful in nutraceutical and pharmaceutical industries.
Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds.[Pubmed:29876936]
J Sci Food Agric. 2019 Jan 15;99(1):302-308.
BACKGROUND: This study aimed to evaluate the presence and content of selected phytochemicals, namely glucosinolates, fatty acids and phenolic compounds, in rutabaga (Brassica napus L. var. napobrassica) sprouts grown under various light conditions, in comparison to rutabaga seeds and roots. As rutabaga sprouts are likely to become new functional food, special emphasis was placed on the related risks of Progoitrin and erucic acid presence - compounds with proven antinutritive properties. RESULTS: Time of sprouting significantly decreased Progoitrin content, especially after 10 days (by 91.5%) and 12 days (by 97.5%), as compared to 8 days. In addition, sprouts grown under dark conditions showed 27%, 60% and 17% reduction in Progoitrin level in 8, 10 and 12 days after sowing, respectively, as compared to sprouts grown under natural conditions. Progoitrin was found to be the predominant glucosinolate in rutabaga seeds (804.07 +/- 60.89 mg 100 g(-1) dry weight (DW)), accompanied by glucoerucin (157.82 +/- 21.04 mg 100 g(-1) DW), also found in the roots (82.20 +/- 16.53 mg 100 g(-1) DW). Among the unsaturated fatty acids in rutabaga sprouts, erucic, linoleic, linolenic and gondoic acids decreased significantly, and only oleic acid increased as germination days progressed. The amount of harmful erucic acid in rutabaga sprouts was found to vary between 1.8% and 7%, depending on the day of seeding or light conditions, as compared to 42.5% in the seeds. CONCLUSION: The evaluated rutabaga products showed a wide content range of potentially antinutritive compounds, sprouts having the lowest amounts of erucic acid and Progoitrin. (c) 2018 Society of Chemical Industry.
Separation and Quantification of Four Main Chiral Glucosinolates in Radix Isatidis and Its Granules Using High-Performance Liquid Chromatography/Diode Array Detector Coupled with Circular Dichroism Detection.[Pubmed:29844266]
Molecules. 2018 May 29;23(6). pii: molecules23061305.
As chemical drugs, separation and quantification of the specific enantiomer from the chiral compounds in herbal medicines are becoming more important. To clarify the chemical characterization of chiral glucosinolates-the antiviral active ingredients of Radix Isatidis, an optimized efficient method of HPLC-UV-CD was developed to simultaneously separate and quantify the four main chiral glucosinolates: Progoitrin, epiProgoitrin, and R,S-goitrin. The first step was to determine Progoitrin, epiProgoitrin, and R,S-goitrin using HPLC-UV, and then determine the R-goitrin and S-goitrin by coupling with CD detection. Subsequently, through the linear relations between anisotropy factor (g factor) and the percent optical purity of R-goitrin, the contents of R-goitrin and S-goitrin from the R,S-goitrin mixture were calculated separately. Furthermore, the chemical composition features of the four chiral glucosinolates in 37 samples from crude drugs, decoction pieces, and granules of R. Isatidis were conducted. The total content of the four glucosinolates was obviously higher in crude drugs, and the variance character of each glucosinolate contents was different. In summary, the accurate measurement method reported here allows for better control of the internal quality of R. Isatidis and its granules and provides a powerful approach for the analysis of other chiral components in traditional Chinese medicines.
Effect of different proportion of sulphur treatments on the contents of glucosinolate in kale (Brassica oleracea var. acephala) commonly consumed in Republic of Korea.[Pubmed:29472789]
Saudi J Biol Sci. 2018 Feb;25(2):349-353.
Kale (Brassica oleracea L. Acephala Group) is the rich source of medicinal value sulphur compounds, glucosinolates (GLSs). The aim of this study was to investigate the effect of different proportion of sulphur (S) supplementation levels on the accumulation of GLSs in the leaves of the kale cultivar ('TBC'). High performance liquid chromatography (HPLC) separation method guided to identify and quantify six GSLs including three aliphatic (Progoitrin, sinigrin and gluconapin) and three indolyl (glucobrassicin, 4-methoxyglucobrassicin and neoglucobrasscin) respectively. Analysis of these distinct levels of S supplementation revealed that the accumulation of individual and total GLSs was directly proportional to the S concentration. The maximum levels of total GLSs (26.8 micromol/g DW) and glucobrassicin (9.98 micromol/g DW) were found in lower and upper parts of the leaves supplemented with 1 mM and 2 mM S, respectively. Interestingly, aliphatic GSLs were noted predominant in all the parts (50.1, 59.3 and 56% of total GSLs). Among the aliphatic and indolyl GSLs, sinigrin and glucobrassicin account 35.3 and 30.88% of the total GSLs. From this study, it is concluded that supply of S enhance the GSLs accumulation in kale.
Molecular characterization of glucosinolates and carotenoid biosynthetic genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis).[Pubmed:29379360]
Saudi J Biol Sci. 2018 Jan;25(1):71-82.
The present study aimed to investigate the contents of glucosinolates (GSLs) and carotenoids in eleven varieties of Chinese cabbage in relation to the expression level of the important transcription factors. MS and HPLC analysis identified the presence of 13 GSLs (Progoitrin, sinigrin, glucoalyssin, gluconapoleiferin, gluconapin, glucocochlearin, glucobrassicanapin, glucoerucin, 4-hydroxyglucobrassicin, glucobrassicin, 4-methoxyglucobrassicin, neoglucobrassicin and gluconasturtiin) and four carotenoids (lutein, zeaxanthin, alpha-carotene and beta-carotene). GSL contents were varied among the different cabbage varieties. The total GSL content ranged from 2.7 to 57.88 mumol/g DW. The proportion of gluconapin (54%) and glucobrassicanapin (22%) was higher in all the varieties, respectively. Results documented the variation in total and individual carotenoid contents that have also been observed among different varieties; however, the total carotenoid contents ranged from 289.12 to 1001.41 mg kg(-1) DW (mean 467.66). Interestingly, the proportion of lutein (66.5) and beta-carotene (25.9) were higher than alpha-carotene (5.1) and zeaxanthin (2.5%). Consequently, the expression level of the regulatory gene, MYB28 was higher in 'K0648' and was directly proportional to GSL content. Similarly, the expression levels of 1-PSY were higher in 'K0112'; however, the expression levels of 2-ZDS, 3-LCYB, 4-LCYE, 5-CHXB and 7-NCED genes showed no significant difference. In addition, the correlation between GSL and carotenoid contents and gene expression level showed moderate significant difference in each Chinese cabbage.


