FlurofamideUrease inhibitor CAS# 70788-28-2 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70788-28-2 | SDF | Download SDF |
| PubChem ID | 51173 | Appearance | Powder |
| Formula | C7H9FN3O2P | M.Wt | 217.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO | ||
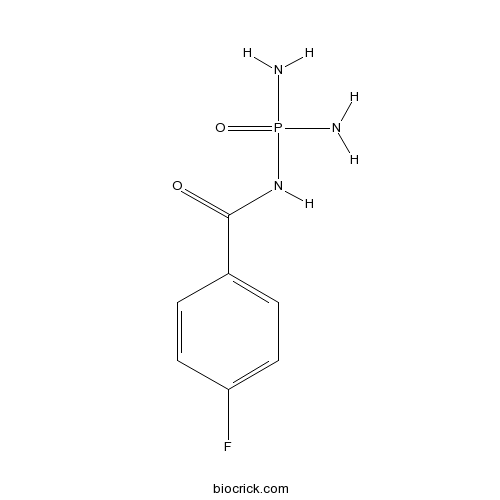
| Chemical Name | N-diaminophosphoryl-4-fluorobenzamide | ||
| SMILES | C1=CC(=CC=C1C(=O)NP(=O)(N)N)F | ||
| Standard InChIKey | QWZFVMCWPLMLTL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H9FN3O2P/c8-6-3-1-5(2-4-6)7(12)11-14(9,10)13/h1-4H,(H5,9,10,11,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Urease inhibitor. |

Flurofamide Dilution Calculator

Flurofamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6053 mL | 23.0266 mL | 46.0532 mL | 92.1065 mL | 115.1331 mL |
| 5 mM | 0.9211 mL | 4.6053 mL | 9.2106 mL | 18.4213 mL | 23.0266 mL |
| 10 mM | 0.4605 mL | 2.3027 mL | 4.6053 mL | 9.2106 mL | 11.5133 mL |
| 50 mM | 0.0921 mL | 0.4605 mL | 0.9211 mL | 1.8421 mL | 2.3027 mL |
| 100 mM | 0.0461 mL | 0.2303 mL | 0.4605 mL | 0.9211 mL | 1.1513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- H-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3323
CAS No.:70702-47-5
- 3-Chlorotyrosine
Catalog No.:BCC2639
CAS No.:70680-93-2
- Pimavanserin
Catalog No.:BCC8065
CAS No.:706779-91-1
- Canniprene
Catalog No.:BCN4271
CAS No.:70677-47-3
- Catharanthine Sulfate
Catalog No.:BCN3859
CAS No.:70674-90-7
- Z-D-Lys-OH
Catalog No.:BCC2761
CAS No.:70671-54-4
- Anisotropine Methylbromide; Octatropine Methylbromide
Catalog No.:BCC8120
CAS No.:70642-90-9
- Boc-D-Tyr-OH
Catalog No.:BCC3463
CAS No.:70642-86-3
- Phlorizin dihydrate
Catalog No.:BCN2584
CAS No.:7061-54-3
- 14beta-Benzoyloxy-2-deacetylbaccatin VI
Catalog No.:BCN1373
CAS No.:705973-69-9
- 19alpha-Hydroxyfern-7-ene
Catalog No.:BCN7405
CAS No.:70588-12-4
- Chrysoobtusin
Catalog No.:BCC8309
CAS No.:70588-06-6
- 2',3'-Dihydroxy-4'-methoxyacetophenone
Catalog No.:BCN7168
CAS No.:708-53-2
- Doxapram hydrochloride monohydrate
Catalog No.:BCC8953
CAS No.:7081-53-0
- FPH1 (BRD-6125)
Catalog No.:BCC5342
CAS No.:708219-39-0
- Panaxydiol
Catalog No.:BCN3702
CAS No.:708257-91-4
- JNJ 10397049
Catalog No.:BCC6139
CAS No.:708275-58-5
- Chicoric acid
Catalog No.:BCN1215
CAS No.:70831-56-0
- Cyanidin-3-O-glucoside chloride
Catalog No.:BCN1230
CAS No.:7084-24-4
- Chlorothiazide Sodium
Catalog No.:BCC5628
CAS No.:7085-44-1
- Troxerutin
Catalog No.:BCN3828
CAS No.:7085-55-4
- (2S)-Isoxanthohumol
Catalog No.:BCN2892
CAS No.:70872-29-6
- Dihydrocitflavanone
Catalog No.:BCN6873
CAS No.:70897-14-2
- SirReal2
Catalog No.:BCC6513
CAS No.:709002-46-0
The failure of flurofamide to control ureaplasma in bovine semen.[Pubmed:3756683]
Can J Vet Res. 1986 Apr;50(2):289-90.
The Flurofamide sensitivities of 21 bovine ureaplasma isolates were determined using the metabolic inhibition method. The 21 isolates included seven each of vaginal, preputial and seminal origin. The minimum inhibitory concentrations of Flurofamide ranged from 0.0125 to 0.2 meg/mL against a ureaplasma titer of 10(4) organisms/mL. The minimum lethal concentrations ranged from less than or equal to 0.1 to 3.2 mcg/mL. Flurofamide was then evaluated in a system comparable to the procedure for semen extension with respect to temperature, time and dilution. The compound was found to be ineffective in reducing ureaplasma numbers in this system at levels up to 1500 mcg/mL.
Ureaplasma-urealyticum-induced bladder stones in rats and their prevention by flurofamide and doxycycline.[Pubmed:3667226]
Isr J Med Sci. 1987 Jun;23(6):565-7.
Struvite calculi can be produced in the bladder of Sprague-Dawley male rats after injection of ureaplasmas into the renal medulla. Calculi appear 3 to 6 days after ureaplasma injection. We have studied the inhibitory effect of Flurofamide, a potent inhibitor of Ureaplasma urealyticum urease, and doxycycline, on the formation of bladder stones. Flurofamide given orally in five doses (total 125 mg) over 3 days and doxycycline in seven doses (total 20 mg) over 4 days partially prevented stone formation only when given at the time of inoculation. Ureaplasmas disappeared rapidly from the urine. The inhibitory effect of Flurofamide was higher than that of doxycycline. However, doxycycline seemed to be efficient when given for a long period (5 weeks).
Marked reduction of Helicobacter pylori-induced gastritis by urease inhibitors, acetohydroxamic acid and flurofamide, in Mongolian gerbils.[Pubmed:11453654]
Biochem Biophys Res Commun. 2001 Jul 20;285(3):728-33.
Urease has been suggested to be essential for colonization and pathogenesis of Helicobacter pylori infection. In the present study, we evaluated the effects of urease inhibitors [acetohydroxamic acid (AHA) and Flurofamide (FFA)] on H. pylori-induced gastritis in Mongolian gerbils. Animals were orally inoculated with H. pylori, and given urease inhibitors in their diet throughout the experimental period of six weeks or four weeks, starting from two weeks after H. pylori inoculation. With the administration of AHA at doses of 100, 500, and 2500 ppm throughout the experimental period, H. pylori-induced gastritis in animals was decreased in a dose-dependent manner, significantly so at 2500 ppm. Suppression of gastric lesions was also evident in animals administered 2500 ppm AHA after the H. pylori infection. Bacterial infection rates were reduced to 40-50% of the control value of 100%, by the highest dose of AHA. The potent urease inhibitor, FFA, also caused marked amelioration of H. pylori-associated gastritis on administration at 100 ppm throughout the six-week experimental period or for four weeks after H. pylori infection. Animals treated with FFA had few visible gastric lesions, and the proportion infected with H. pylori was reduced to less than 10%. Since antibiotic-resistant strains of H. pylori have become a serious problem, nonantibiotic urease inhibitors may be very useful to control H. pylori-associated gastroduodenal disease.
Investigation into the inhibitory effect of flurofamide on animal ureaplasmas and its use in the treatment of ureaplasma-infected sheep.[Pubmed:3761418]
J Vet Pharmacol Ther. 1986 Sep;9(3):280-5.
In vitro tests with the urease inhibitor Flurofamide demonstrated that the final inhibitory concentration of 0.5-4 microM on the growth of nine ureaplasma strains was largely ureaplasmastatic, requiring prolonged incubation to have a ureaplasmacidal effect. Intramuscular injection of Flurofamide successfully eliminated genital infections of ureaplasma in sheep only when the treatment was repeated on two consecutive days. A dose rate of 5-20 mg/kg body weight eliminated the organism from naturally infected sheep, but 15-25 mg/kg body weight was required to eliminate the infection from eleven of fourteen experimentally, newly infected sheep. Administration of the Flurofamide orally in the drinking water failed to eliminate ureaplasmas from any of twenty newly infected sheep.


