Chicoric acidCAS# 70831-56-0 |
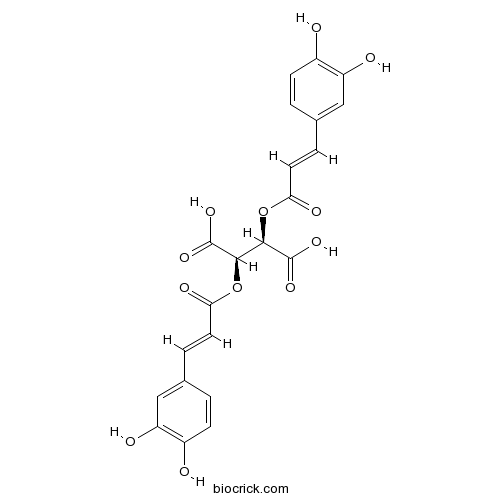
- D-Chicoric Acid
Catalog No.:BCC8148
CAS No.:52248-48-3
- Cichoric acid
Catalog No.:BCN9033
CAS No.:6537-80-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 70831-56-0 | SDF | Download SDF |
| PubChem ID | 5281764 | Appearance | White powder |
| Formula | C22H18O12 | M.Wt | 474.37 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | (-)-Chicoric acid; trans-Caffeoyltartaric acid;L-Chicoric Acid;6537-80-0 | ||
| Solubility | DMSO : 100 mg/mL (210.81 mM; Need ultrasonic) | ||
| Chemical Name | (2R,3R)-2,3-bis[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy]butanedioic acid | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)OC(C(C(=O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)C(=O)O)O)O | ||
| Standard InChIKey | YDDGKXBLOXEEMN-IABMMNSOSA-N | ||
| Standard InChI | InChI=1S/C22H18O12/c23-13-5-1-11(9-15(13)25)3-7-17(27)33-19(21(29)30)20(22(31)32)34-18(28)8-4-12-2-6-14(24)16(26)10-12/h1-10,19-20,23-26H,(H,29,30)(H,31,32)/b7-3+,8-4+/t19-,20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chicoric acid, a new compound able to enhance insulin release and glucose uptake, it is a new potential antidiabetic agent carrying both insulin sensitizing and insulin-secreting properties. Chicoric acid has antiobesity effects, it can induce apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. L-Chicoric acid has antiviral activity against HIV-1, which has been attributed to the inhibition of HIV-1 integration. |
| Targets | TLR | NOS | PARP | MMP(e.g.TIMP) | Bcl-2/Bax | Caspase | p38MAPK | JNK | ERK | HO-1 | COX | Akt | PI3K | HIV | NO | PGE | NF-kB | TNF-α |
| In vitro | Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways.[Pubmed: 23363008]J Agric Food Chem. 2013 Feb 20;61(7):1509-20.Chicoric acid has been reported to possess various bioactivities. However, the antiobesity effects of Chicoric acid remain poorly understood.
Chicoric acid, a new compound able to enhance insulin release and glucose uptake.[Pubmed: 18834859 ]Biochem Biophys Res Commun. 2008 Dec 5;377(1):131-5.Caffeic acid and chlorogenic acid (CGA), a mono-caffeoyl ester, have been described as potential antidiabetic agents.
|
| In vivo | Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study.[Pubmed: 24418658]Fitoterapia. 2014 Mar;93:132-41.Ocimum gratissimum L. is popularly used to treat diabetes mellitus. The hypoglycemic activity of this medicinal species has been confirmed by in vivo studies.
|
| Kinase Assay | Viral entry as the primary target for the anti-HIV activity of chicoric acid and its tetra-acetyl esters.[Pubmed: 10953059]Mol Pharmacol. 2000 Sep;58(3):641-8.The antiviral activity of L-Chicoric acid against HIV-1 has been attributed previously to the inhibition of HIV-1 integration. This conclusion was based on the inhibition of integrase activity in enzymatic assays and the isolation of a resistant HIV strain with a mutation (G140S) in the integrase gene.
|
| Cell Research | Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells.[Pubmed: 21513709 ]Eur J Pharmacol. 2011 Jun 25;660(2-3):454-9.Synergistic anti-inflammatory effects of luteolin and Chicoric acid, two abundant constituents of the common dandelion (Taraxacum officinale Weber), were investigated in lipopolysaccharide (LPS) stimulated RAW 264.7 cells.
|
| Animal Research | Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice.[Pubmed: 24985007]Nutrition. 2014 Jul-Aug;30(7-8):882-9.Acute and chronic consumption of alcohol can alter intestinal barrier function thereby increasing portal endotoxin levels subsequently leading to an activation of toll-like receptor (TLR) 4-dependent signaling cascades, elevated levels of reactive oxygen species and induction of tumor necrosis factor α in the liver. Recent studies suggest that Chicoric acid found in Echinacea pupurea, chicory, and other plants, may possess antioxidant and anti-inflammatory effects. The aim of the present study was to determine if Chicoric acid can reduce acute alcohol-induced liver damage.
|

Chicoric acid Dilution Calculator

Chicoric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1081 mL | 10.5403 mL | 21.0806 mL | 42.1612 mL | 52.7015 mL |
| 5 mM | 0.4216 mL | 2.1081 mL | 4.2161 mL | 8.4322 mL | 10.5403 mL |
| 10 mM | 0.2108 mL | 1.054 mL | 2.1081 mL | 4.2161 mL | 5.2701 mL |
| 50 mM | 0.0422 mL | 0.2108 mL | 0.4216 mL | 0.8432 mL | 1.054 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2108 mL | 0.4216 mL | 0.527 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
L-Chicoric acid is an inhibitor of human immunodeficiency virus type 1 (HIV-1) integrase in vitro and of HIV-1 replication in tissue culture. IC50 value: Target: In vitro: Using quantitative real-time polymerase chain reaction (PCR), l-CA inhibits integration at concentrations from 500 nM to 10 μM but also inhibits entry at concentrations above 1 μM [1]. l-Chicoric acid, an inhibitor of human immunodeficiency virus type 1 (HIV-1) integrase, improves on the in vitro anti-HIV-1 effect of Zidovudine plus a protease inhibitor (AG1350) [2]. L-chicoric acid inhibits integrase and that the drug is likely to interact at residues near the catalytic triad in the integrase active site [3]. In vivo:
References:
[1]. Ryan A Reinke, et al. l-Chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology Volume 326, Issue 2, 1 September 2004, Pages 203–219
[2]. W Edward Robinson Jr., et al. l-Chicoric acid, an inhibitor of human immunodeficiency virus type 1 (HIV-1) integrase, improves on the in vitro anti-HIV-1 effect of Zidovudine plus a protease inhibitor (AG1350). Antiviral Research Volume 39, Issue 2, Augu
[3]. King PJ, et al. Resistance to the anti-human immunodeficiency virus type 1 compound L-chicoric acid results from a single mutation at amino acid 140 of integrase. Journal of Virology, 1998, 72(10): 8420-8424
- JNJ 10397049
Catalog No.:BCC6139
CAS No.:708275-58-5
- Panaxydiol
Catalog No.:BCN3702
CAS No.:708257-91-4
- FPH1 (BRD-6125)
Catalog No.:BCC5342
CAS No.:708219-39-0
- Doxapram hydrochloride monohydrate
Catalog No.:BCC8953
CAS No.:7081-53-0
- 2',3'-Dihydroxy-4'-methoxyacetophenone
Catalog No.:BCN7168
CAS No.:708-53-2
- Flurofamide
Catalog No.:BCC5660
CAS No.:70788-28-2
- H-D-Ala(3-pyridyl)-OH.HCl
Catalog No.:BCC3323
CAS No.:70702-47-5
- 3-Chlorotyrosine
Catalog No.:BCC2639
CAS No.:70680-93-2
- Pimavanserin
Catalog No.:BCC8065
CAS No.:706779-91-1
- Canniprene
Catalog No.:BCN4271
CAS No.:70677-47-3
- Catharanthine Sulfate
Catalog No.:BCN3859
CAS No.:70674-90-7
- Z-D-Lys-OH
Catalog No.:BCC2761
CAS No.:70671-54-4
- Cyanidin-3-O-glucoside chloride
Catalog No.:BCN1230
CAS No.:7084-24-4
- Chlorothiazide Sodium
Catalog No.:BCC5628
CAS No.:7085-44-1
- Troxerutin
Catalog No.:BCN3828
CAS No.:7085-55-4
- (2S)-Isoxanthohumol
Catalog No.:BCN2892
CAS No.:70872-29-6
- Dihydrocitflavanone
Catalog No.:BCN6873
CAS No.:70897-14-2
- SirReal2
Catalog No.:BCC6513
CAS No.:709002-46-0
- 2-(5-Chloro-2-phenoxyphenyl)acetic acid
Catalog No.:BCC8481
CAS No.:70958-20-2
- 7,3',4'-Tri-O-methyleriodictyol
Catalog No.:BCN7766
CAS No.:70987-96-1
- H-His-OH
Catalog No.:BCC2954
CAS No.:71-00-1
- Cytosine
Catalog No.:BCN8533
CAS No.:71-30-7
- butanol
Catalog No.:BCN4976
CAS No.:71-36-3
- Medroxyprogesterone acetate
Catalog No.:BCC4485
CAS No.:71-58-9
Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-kappaB translocation in LPS stimulated RAW 264.7 cells.[Pubmed:21513709]
Eur J Pharmacol. 2011 Jun 25;660(2-3):454-9.
Synergistic anti-inflammatory effects of luteolin and Chicoric acid, two abundant constituents of the common dandelion (Taraxacum officinale Weber), were investigated in lipopolysaccharide (LPS) stimulated RAW 264.7 cells. Co-treatment with luteolin and Chicoric acid synergistically reduced cellular concentrations of nitric oxide (NO) and prostaglandin E2 (PGE2) and also inhibited expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). In addition, co-treatment reduced the levels of proinflammatory cytokines, tumor necrosis factor (TNF)-alpha, and interleukin (IL)-1beta. Both luteolin and Chicoric acid suppressed oxidative stress, but they did not exhibit any synergistic activity. Luteolin and Chicoric acid co-treatment inhibited phosphorylation of NF-kappaB and Akt, but had no effect on extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p38. This anti-inflammatory signaling cascade coincides with that affected by luteolin treatment alone. These results suggest that luteolin plays a central role in ameliorating LPS-induced inflammatory cascades via inactivation of the NF-kappaB and Akt pathways, and that Chicoric acid strengthens the anti-inflammatory activity of luteolin through NF-kappaB attenuation.
Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study.[Pubmed:24418658]
Fitoterapia. 2014 Mar;93:132-41.
Ocimum gratissimum L. is popularly used to treat diabetes mellitus. The hypoglycemic activity of this medicinal species has been confirmed by in vivo studies. The present study conducted a chemical investigation of a leaf decoction (10% p/v) of O. gratissimum monitored by in vivo hypoglycemic activity assays. Four phenolic substances were identified: L-caftaric acid (1), L-Chicoric acid (2), eugenyl-beta-D-glucopyranoside (3) and vicenin-2 (4). The acute hypoglycemic activity of the O. gratissimum decoction fractions Og1-S (300 mg/kg), Og1-A (240 mg/kg) and Og1-B (80 mg/kg) was evaluated intraperitoneally in normal and streptozotocin-induced diabetic mice. They reduced glycemia by 63%, 76% and 60% (in 120 min), respectively, in the diabetic mice. Subfractions of Og1-A were also evaluated under the same conditions: Og1-AS (200 mg/kg) and Og1-AP (40 mg/kg) produced a decrease of only 37% and 39%, respectively. Among the major phenolic substances, only Chicoric acid (2; 3 mg/kg) reduced significantly the glycemic levels of diabetic mice by 53%, 120 min after treatment. This is the first study describing the hypoglycemic activity of Chicoric acid in an animal model of diabetes mellitus. In addition, we suggest that there may be other substances contributing to this activity. Thus, for the first time, a correlation is established between the hypoglycemic activity of O. gratissimum and its chemical composition.
Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways.[Pubmed:23363008]
J Agric Food Chem. 2013 Feb 20;61(7):1509-20.
Chicoric acid has been reported to possess various bioactivities. However, the antiobesity effects of Chicoric acid remain poorly understood. In this study, we investigated the effects of Chicoric acid on 3T3-L1 preadipocytes and its molecular mechanisms of apoptosis. Chicoric acid inhibited cell viability and induced apoptosis in 3T3-L1 preadipocytes which was characterized by chromatin condensation and poly ADP-ribose-polymerase (PARP) cleavage. Mitochondrial membrane potential (MMP) loss, Bax/Bcl-2 dysregulation, cytochrome c release, and caspase-3 activation were observed, indicating mitochondria-dependent apoptosis induced by Chicoric acid. Furthermore, PI3K/Akt and MAPK (p38 MAPK, JNK, and ERK1/2) signaling pathways were involved in Chicoric acid-induced apoptosis. The employment of protein kinase inhibitors LY294002, SB203580, SP600125, and U0126 revealed that PI3K/Akt signaling pathway interplayed with MAPK signaling pathways. Moreover, Chicoric acid induced reactive oxygen species (ROS) generation. Pretreatment with the antioxidant N-acetylcysteine (NAC) significantly blocked cell death and changes of Akt and MAPK signalings induced by Chicoric acid. In addition, Chicoric acid down regulated HO-1 and COX-2 via the PI3K/Akt pathway.
Oral intake of chicoric acid reduces acute alcohol-induced hepatic steatosis in mice.[Pubmed:24985007]
Nutrition. 2014 Jul-Aug;30(7-8):882-9.
OBJECTIVE: Acute and chronic consumption of alcohol can alter intestinal barrier function thereby increasing portal endotoxin levels subsequently leading to an activation of toll-like receptor (TLR) 4-dependent signaling cascades, elevated levels of reactive oxygen species and induction of tumor necrosis factor alpha in the liver. Recent studies suggest that Chicoric acid found in Echinacea pupurea, chicory, and other plants, may possess antioxidant and anti-inflammatory effects. The aim of the present study was to determine if Chicoric acid can reduce acute alcohol-induced liver damage. METHODS: Female mice were given Chicoric acid orally (4 mg/kg body weight) for 4 d before acute ethanol administration (6 g/kg body weight). Furthermore, the effect of Chicoric acid on the lipopolysaccharide (LPS)-dependent activation in an in vitro model of Kupffer cells (RAW264.7 macrophages) was assessed. RESULTS: Acute alcohol ingestion caused a significant increase in hepatic triacylglycerols accumulation, which was associated with increased protein levels of the inducible nitric oxide synthase (iNOS), 4-hydroxynonenal protein adducts, and active plasminogen activator inhibitor 1 protein in the liver. Pretreatment of animals with Chicoric acid significantly attenuated these effects of alcohol on the liver. In LPS-treated RAW264.7 macrophages, pretreatment with Chicoric acid significantly suppressed LPS-induced mRNA expression of iNOS and tumor necrosis factor alpha. CONCLUSION: These data suggest that Chicoric acid may reduce acute alcohol-induced steatosis in mice through interfering with the induction of iNOS and iNOS-dependent signaling cascades in the liver.
Viral entry as the primary target for the anti-HIV activity of chicoric acid and its tetra-acetyl esters.[Pubmed:10953059]
Mol Pharmacol. 2000 Sep;58(3):641-8.
The antiviral activity of L-Chicoric acid against HIV-1 has been attributed previously to the inhibition of HIV-1 integration. This conclusion was based on the inhibition of integrase activity in enzymatic assays and the isolation of a resistant HIV strain with a mutation (G140S) in the integrase gene. Here we show that the primary antiviral target of L-CA and its analogs in cell culture is viral entry. L- and D-Chicoric acid (L-CA and D-CA) and their respective tetra-acetyl esters inhibit the replication of HIV-1 (III(B) and NL4.3) and HIV-2 (ROD) in MT-4 cells at a 50% effective concentration (EC(50)) ranging from 1.7 to 70.6 microM. In a time-of-addition experiment, L-CA, D-CA, L-CATA, and D-CATA were found to interfere with an early event in the viral replication cycle. Moreover, L-CA, D-CA, and their analogs did not inhibit the replication of virus strains that were resistant toward polyanionic and polycationic compounds at subtoxic concentrations. Furthermore, HIV-1 strains resistant to L-CA and D-CA were selected in the presence of L-CA and D-CA, respectively. Mutations were found in the V2, V3, and V4 loop region of the envelope glycoprotein gp120 of the L-CA and D-CA-resistant NL4.3 strains that were not present in the wild-type NL4.3 strain. Recombination of the gp120 gene of the L-CA and D-CA resistant strain in a NL4.3 wild-type molecular clone fully rescued the phenotypic resistance toward L-CA and D-CA. No significant mutations were detected in the integrase gene of the drug-resistant virus strains. Although inhibition of HIV integrase activity by L-CA and its derivatives was confirmed in an oligonucleotide-driven assay, integrase carrying the G140S mutation was inhibited to the same extent as the wild-type integrase.
Chicoric acid, a new compound able to enhance insulin release and glucose uptake.[Pubmed:18834859]
Biochem Biophys Res Commun. 2008 Dec 5;377(1):131-5.
Caffeic acid and chlorogenic acid (CGA), a mono-caffeoyl ester, have been described as potential antidiabetic agents. Using in vitro studies, we report the effects of a dicaffeoyl ester, Chicoric acid (CRA) purified from Cichorium intybus, on glucose uptake and insulin secretion. Our results show that CRA and CGA increased glucose uptake in L6 muscular cells, an effect only observed in the presence of stimulating concentrations of insulin. Moreover, we found that both CRA and CGA were able to stimulate insulin secretion from the INS-1E insulin-secreting cell line and rat islets of Langerhans. In the later case, the effect of CRA is only observed in the presence of subnormal glucose levels. Patch clamps studies show that the mechanism of CRA and CGA was different from that of sulfonylureas, as they did not close K(ATP) channels. Chicoric acid is a new potential antidiabetic agent carrying both insulin sensitizing and insulin-secreting properties.


