Glucosamine sulfateCAS# 29031-19-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29031-19-4 | SDF | Download SDF |
| PubChem ID | 115046 | Appearance | Powder |
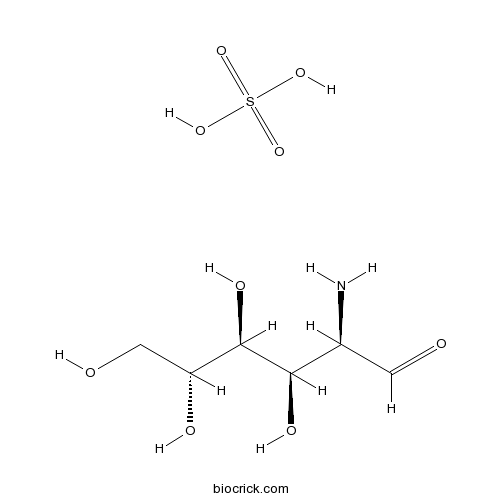
| Formula | C6H15NO9S | M.Wt | 277.25 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4S,5R)-2-amino-3,4,5,6-tetrahydroxyhexanal;sulfuric acid | ||
| SMILES | C(C(C(C(C(C=O)N)O)O)O)O.OS(=O)(=O)O | ||
| Standard InChIKey | FGNPLIQZJCYWLE-BTVCFUMJSA-N | ||
| Standard InChI | InChI=1S/C6H13NO5.H2O4S/c7-3(1-8)5(11)6(12)4(10)2-9;1-5(2,3)4/h1,3-6,9-12H,2,7H2;(H2,1,2,3,4)/t3-,4+,5+,6+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glucosamine sulfate has environmental antibacterial activity. Glucosamine sulfate could as a safe symptomatic Slow Acting Drug for osteoarthritis, it can stimulate proteoglycan synthesis by chondrocytes and has mild anti-inflammatory properties, it is therefore as effective as ibuprofen on symptoms of knee osteoarthritis. |
| Targets | IL Receptor | MMP(e.g.TIMP) | Antifection |
| In vitro | The effects of glucosamine sulfate on intervertebral disc annulus fibrosus cells in vitro.[Pubmed: 24361347]Spine J. 2013 Dec 18. pii: S1529-9430(13)01987-6Glucosamine has gained widespread use among patients, despite inconclusive efficacy data. Inconsistency in the clinical literature may be related to lack of understanding of the effects of glucosamine on the intervertebral disc, and therefore, improper patient selection.
The goal of our study was to investigate the effects of glucosamine on intervertebral disc cells in vitro under the physiological conditions of inflammation and mechanical loading.
Controlled in vitro laboratory setting.
Glucosamine sulfate--environmental antibacterial activity.[Pubmed: 19495827]Clin Rheumatol. 2009 Oct;28(10):1221-3.We have recently showed antibacterial activity against E. coli in vitro of a trademark Mega-Gluflex-containing Glucosamine sulfate (GS) and chondroitin sulfate (CS). The purpose of this study was to examine the antibacterial activity of Glucosamine sulfate as a new trademark Arthryl in vitro. |
| In vivo | Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee.[Pubmed: 11548225]Osteoarthritis Cartilage. 1994 Mar;2(1):61-9.Glucosamine sulfate is able to stimulate proteoglycan synthesis by chondrocytes and has mild anti-inflammatory properties. In clinical trials, Glucosamine sulfate was more effective than placebo in controlling the symptoms of osteoarthritis (OA). |
| Animal Research | Glucosamine-sulfate on fracture healing.[Pubmed: 23588972]Ulus Travma Acil Cerrahi Derg. 2013 Jan;19(1):8-12.The aim of this study is to determine whether glucosamine-sulfate has any effects on bone-healing.
|

Glucosamine sulfate Dilution Calculator

Glucosamine sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6069 mL | 18.0343 mL | 36.0685 mL | 72.1371 mL | 90.1713 mL |
| 5 mM | 0.7214 mL | 3.6069 mL | 7.2137 mL | 14.4274 mL | 18.0343 mL |
| 10 mM | 0.3607 mL | 1.8034 mL | 3.6069 mL | 7.2137 mL | 9.0171 mL |
| 50 mM | 0.0721 mL | 0.3607 mL | 0.7214 mL | 1.4427 mL | 1.8034 mL |
| 100 mM | 0.0361 mL | 0.1803 mL | 0.3607 mL | 0.7214 mL | 0.9017 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- 3-Epiglochidiol
Catalog No.:BCN5193
CAS No.:29028-10-2
- Fmoc-Gly-OH
Catalog No.:BCC3498
CAS No.:29022-11-5
- Benzoyl-DL-valine
Catalog No.:BCC8864
CAS No.:2901-80-6
- Methyl reserpate
Catalog No.:BCN3489
CAS No.:2901-66-8
- Boc-Phg-OH
Catalog No.:BCC3311
CAS No.:2900-27-8
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
- 3-(Benzylthio)-Propionic acid
Catalog No.:BCC2839
CAS No.:2899-66-3
- H-Methioninol
Catalog No.:BCC2721
CAS No.:2899-37-8
- H-Tryptophanol
Catalog No.:BCC2701
CAS No.:2899-29-8
- H-Trp-Oet.HCl
Catalog No.:BCC2673
CAS No.:2899-28-7
- Z-N-Me-Phe-OH
Catalog No.:BCC3349
CAS No.:2899-07-2
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Bis(2-carboxyethyl)isocyanurate
Catalog No.:BCC8880
CAS No.:2904-40-7
- Matricin
Catalog No.:BCC8209
CAS No.:29041-35-8
- Hexanorcucurbitacin D
Catalog No.:BCN7875
CAS No.:29065-05-2
- Pachymic acid
Catalog No.:BCN6347
CAS No.:29070-92-6
- 5-Hydroxy-3',4',7-trimethoxyflavone
Catalog No.:BCN5194
CAS No.:29080-58-8
- Eupteleasaponin I
Catalog No.:BCN7839
CAS No.:290809-29-9
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
- Glipizide
Catalog No.:BCC3785
CAS No.:29094-61-9
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
The effects of glucosamine sulfate on intervertebral disc annulus fibrosus cells in vitro.[Pubmed:24361347]
Spine J. 2015 Jun 1;15(6):1339-46.
BACKGROUND CONTEXT: Glucosamine has gained widespread use among patients, despite inconclusive efficacy data. Inconsistency in the clinical literature may be related to lack of understanding of the effects of glucosamine on the intervertebral disc, and therefore, improper patient selection. PURPOSE: The goal of our study was to investigate the effects of glucosamine on intervertebral disc cells in vitro under the physiological conditions of inflammation and mechanical loading. STUDY DESIGN: Controlled in vitro laboratory setting. METHODS: Intervertebral disc cells isolated from the rabbit annulus fibrosus were exposed to Glucosamine sulfate in the presence and absence of interleukin-1beta and tensile strain. Outcome measures included gene expression, measurement of total glycosaminoglycans, new proteoglycan synthesis, prostaglandin E2 production, and matrix metalloproteinase activity. The study was funded by NIH/NCCAM, and the authors have no conflicts of interest. RESULTS: Under conditions of inflammatory stimulation alone, glucosamine demonstrated a dose-dependent effect in decreasing inflammatory and catabolic mediators and increasing anabolic genes. However, under conditions of mechanical stimulation, although inflammatory gene expression was decreased, PGE2 was not. In addition, matrix metalloproteinase-3 gene expression was increased and aggrecan expression decreased, both of which would have a detrimental effect on matrix homeostasis. Consistent with this, measurement of total glycosaminoglycans and new proteoglycan synthesis demonstrated detrimental effects of glucosamine under all conditions tested. CONCLUSIONS: These results may in part help to explain the conflicting reports of efficacy, as there is biological plausibility for a therapeutic effect under conditions of predominate inflammation but not under conditions where mechanical loading is present or in which matrix synthesis is needed.
Glucosamine sulfate--environmental antibacterial activity.[Pubmed:19495827]
Clin Rheumatol. 2009 Oct;28(10):1221-3.
We have recently showed antibacterial activity against E. coli in vitro of a trademark Mega-Gluflex-containing Glucosamine sulfate (GS) and chondroitin sulfate (CS). The purpose of this study was to examine the antibacterial activity of GS as a new trademark Arthryl (Manufacturer Rottapharm Ltd, Ireland; Distributor in Israel Rafa Laboratories Ltd) in vitro. We used cabbage and chicken broths and milk (every media of 20 ml) left opened for 1 week with and without Arthryl supplements 1,500 mg, the content of one package of the medication. A similar volume (20 ml) is ingested in taking the medication. Experiments with three repeatable results were taken for consideration. Arthryl inhibited environmental bacterial colonies' growth in every media but fungi growth was not impaired. Milk stayed liquid for the whole week with supplement of the Arthryl compared with sour milk transformation without Arthryl. Sample B showed inhibitory properties of the bacterial colonies on the fungi growth. The sample with Arthryl showed progressive growth of fungi without bacterial growth after 10 days of follow up compared with bacterial growth on media without Arthryl. Glucosamine sulfate as a new trademark Arthryl has environmental antibacterial properties but does not inhibit growth of fungal colonies.
Glucosamine-sulfate on fracture healing.[Pubmed:23588972]
Ulus Travma Acil Cerrahi Derg. 2013 Jan;19(1):8-12.
BACKGROUND: The aim of this study is to determine whether glucosamine-sulfate has any effects on bone-healing. METHODS: A unilateral fracture was created in the tibia of sixty-one female rats. Rats were given no drug or 230 mg/kg glucosamine-sulfate daily. Fractures were analyzed during the first, second and fourth weeks after creation of fracture. Quantitative measurement for new bone formation and osteoblast lining were determined histologically. Semiquantitative score for fracture healing was used for histomorphometric analyses. Bridging bone formation was assessed radiographically. RESULTS: New bone formation and osteoblast lining were significantly higher in glucosamine-treated group at week 1. Surrounding connective tissue was more cellular and vascular, and the newly formed bone trabecules were present in greater amounts in glucosamine-treated group, compared to control group at week 1 and 4. But radiologically, the control group had better scores than that of the glucosamine-treated group at week 4. CONCLUSION: These data demonstrate that daily glucosamine-sulfate administration accelerates early phase of fracture repair in the rat tibia, with increased new bone formation and osteoblast lining histologically, but radiologic bone union is not favored on radiographs.
Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee.[Pubmed:11548225]
Osteoarthritis Cartilage. 1994 Mar;2(1):61-9.
Glucosamine sulfate is able to stimulate proteoglycan synthesis by chondrocytes and has mild anti-inflammatory properties. In clinical trials, Glucosamine sulfate was more effective than placebo in controlling the symptoms of osteoarthritis (OA). In order to better characterize this therapeutic activity, we conducted a randomized, double-blind, parallel-group study of Glucosamine sulfate 500 mg t.i.d. vs ibuprofen 400 mg t.i.d., orally for 4 weeks. The study included 200 hospitalized patients with active OA of the knee, symptoms for at least 3 months and a Lequesne's index of at least 7 points. Patients were evaluated weekly. Response was defined as a reduction in the Lequesne's index by at least 2 points if the enrollment value was higher than 12 points, or by at least 1 point if the enrollment value was 12 or less points, together with a positive overall assessment by the investigator. The improvement tended to be sooner under ibuprofen (48% responders vs 28% after the 1st treatment week; P = 0.06, Fisher's Exact test), but there was no difference from the 2nd week onward, with a success rate of 52% in the ibuprofen group and of 48% in the glucosamine group (P = 0.67) at the end of treatment. The average Lequesne's index at enrollment was around 16 points and decreased by over 6 points in both groups, again with the above described trend. On the other hand, 35% of patients on ibuprofen reported adverse events, mainly of gastrointestinal origin, vs 6% adverse events with glucosamine (P < 0.001, Fisher's Exact test). The number of adverse event related drop-outs was different between the two groups (7% vs 1%, respectively; P = 0.035). Glucosamine sulfate was therefore as effective as ibuprofen on symptoms of knee OA. These data confirm Glucosamine sulfate as a safe symptomatic Slow Acting Drug for OA.


