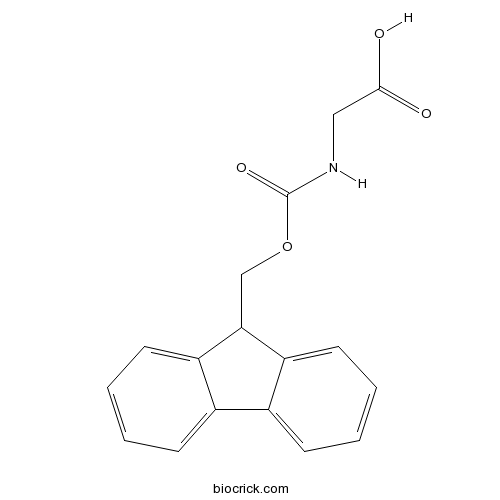
Fmoc-Gly-OHCAS# 29022-11-5 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29022-11-5 | SDF | Download SDF |
| PubChem ID | 93124 | Appearance | Powder |
| Formula | C17H15NO4 | M.Wt | 297.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(9H-fluoren-9-ylmethoxycarbonylamino)acetic acid | ||
| SMILES | C1=CC=C2C(=C1)C(C3=CC=CC=C32)COC(=O)NCC(=O)O | ||
| Standard InChIKey | NDKDFTQNXLHCGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H15NO4/c19-16(20)9-18-17(21)22-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15H,9-10H2,(H,18,21)(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Gly-OH Dilution Calculator

Fmoc-Gly-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3636 mL | 16.818 mL | 33.6361 mL | 67.2721 mL | 84.0901 mL |
| 5 mM | 0.6727 mL | 3.3636 mL | 6.7272 mL | 13.4544 mL | 16.818 mL |
| 10 mM | 0.3364 mL | 1.6818 mL | 3.3636 mL | 6.7272 mL | 8.409 mL |
| 50 mM | 0.0673 mL | 0.3364 mL | 0.6727 mL | 1.3454 mL | 1.6818 mL |
| 100 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6727 mL | 0.8409 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Gly-OH
- Benzoyl-DL-valine
Catalog No.:BCC8864
CAS No.:2901-80-6
- Methyl reserpate
Catalog No.:BCN3489
CAS No.:2901-66-8
- Boc-Phg-OH
Catalog No.:BCC3311
CAS No.:2900-27-8
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
- 3-(Benzylthio)-Propionic acid
Catalog No.:BCC2839
CAS No.:2899-66-3
- H-Methioninol
Catalog No.:BCC2721
CAS No.:2899-37-8
- H-Tryptophanol
Catalog No.:BCC2701
CAS No.:2899-29-8
- H-Trp-Oet.HCl
Catalog No.:BCC2673
CAS No.:2899-28-7
- Z-N-Me-Phe-OH
Catalog No.:BCC3349
CAS No.:2899-07-2
- Persicoside
Catalog No.:BCN4780
CAS No.:28978-03-2
- Pectolinarin
Catalog No.:BCN1217
CAS No.:28978-02-1
- CB 300919
Catalog No.:BCC1456
CAS No.:289715-28-2
- 3-Epiglochidiol
Catalog No.:BCN5193
CAS No.:29028-10-2
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- Glucosamine sulfate
Catalog No.:BCN5981
CAS No.:29031-19-4
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Bis(2-carboxyethyl)isocyanurate
Catalog No.:BCC8880
CAS No.:2904-40-7
- Matricin
Catalog No.:BCC8209
CAS No.:29041-35-8
- Hexanorcucurbitacin D
Catalog No.:BCN7875
CAS No.:29065-05-2
- Pachymic acid
Catalog No.:BCN6347
CAS No.:29070-92-6
- 5-Hydroxy-3',4',7-trimethoxyflavone
Catalog No.:BCN5194
CAS No.:29080-58-8
- Eupteleasaponin I
Catalog No.:BCN7839
CAS No.:290809-29-9
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
Peptide ionophores: synthesis and cation-binding properties of a bicyclic peptide containing glycine and lysine residues.[Pubmed:7655187]
Pept Res. 1995 Mar-Apr;8(2):62-9.
Peptide 1, cyclo(1,5-epsilon-succinoyl) (Lys-Gly-Gly-Gly)2, is a representative member of a family of polycyclic peptide ionophores characterized by C2 symmetry and a relatively flexible structure resulting from its high Gly content. Peptide 1 has been synthesized by two different solid-phase protocols from its linear precursors, H-Gly-Gly-Lys(Fmoc)-Gly-Gly-Gly-Lys(Fmoc)-Gly-OH and H-Gly-Gly-Lys(Z)-Gly-Gly-Gly-Lys(Z)-Gly-OH), and satisfactorily characterized by chemical means. The CD spectrum of 1 is compatible with a beta-folded structure, stabilized by two internal hydrogen bonds. The complexation behavior of 1 toward alkaline and alkaline-earth cations can be envisaged as an equilibrium between inclusion (1:1) and sandwich (2:1) complex models, with affinities in the 10(6) M-1 and 10(11) M-2 range, respectively. A slight preference of 1 for Sr2+ over other cations has been found.
Honeycomb membranes prepared from 3-O-amino acid functionalized cellulose derivatives.[Pubmed:24188846]
Carbohydr Polym. 2014 Jan 16;100:126-34.
The development of value-added wood-derived polymer products is of significant importance. Of particular interest is the synthesis of advanced bioactive cellulosic materials. In the present research, novel cellulosic honeycomb films are reported. Cellulose was reacted with dimethylthexylsilyl chloride to form regioselective 2,6-di-O-thexyldimethylsilyl cellulose followed by substitution of the C3 with functionalized poly(ethylene glycol) (PEG). The free end of the PEG side chains of the regioselective 3-O-poly(ethylene glycol)-2,6-di-O-thexyldimethylsilyl cellulose served as an attachment point for bioactive molecules. As an example, Fmoc-Gly-OH was linked to the free end of PEG to produce 3-O-Fmoc-Gly-poly(ethylene glycol)-2,6-di-O-thexyldimethylsilyl cellulose. Honeycomb films were produced through film casting under a humid airflow. AFM analysis revealed the directed self-assembly of the 3-O-Fmoc-Gly-poly(ethylene glycol)-2,6-di-O-thexyldimethylsilyl cellulose wherein the pendent 3-O-Fmoc-Gly-poly(ethylene glycol) groups allocated preferentially around the edges of the honeycomb pores.
Dicyclopropylmethyl peptide backbone protectant.[Pubmed:19719204]
Org Lett. 2009 Aug 20;11(16):3718-21.
The N-dicyclopropylmethyl (Dcpm) residue, introduced into amino acids via reaction of dicyclopropylmethanimine hydrochloride with an amino acid ester followed by sodium cyanoborohydride or triacetoxyborohydride reduction, can be used as an amide bond protectant for peptide synthesis. Examples which demonstrate the amelioration of aggregation effects include syntheses of the alanine decapeptide and the prion peptide (106-126). Avoidance of cyclization to the aminosuccinimide followed substitution of Fmoc-(Dcpm)Gly-OH for Fmoc-Gly-OH in the assembly of sequences containing the sensitive Asp-Gly unit.
'O-Acyl isopeptide method' for peptide synthesis: Solvent effects in the synthesis of Abeta1-42 isopeptide using 'O-acyl isodipeptide unit'.[Pubmed:17803257]
J Pept Sci. 2007 Dec;13(12):868-74.
'O-Acyl isopeptide method' is an efficient synthetic method for peptides. We designed 'O-acyl isodipeptide units', Boc-Ser/Thr(Fmoc-Xaa)-OH, as important building blocks to enable routine use of the O-acyl isopeptide method. In the synthesis of an Abeta1-42 isopeptide using O-acyl isodipeptide unit Boc-Ser(Fmoc-Gly)-OH, a side reaction, resulting in the deletion of Ser(26) in the O-acyl isopeptide structure, was noticed during coupling of the unit. We observed that the side reaction occurred during the activation step and was solvent-dependent. In DMF or NMP, an intramolecular side reaction, originating from the activated species of the unit, occurred during the activation step. In non-polar solvents such as CHCl(3) or CH(2)Cl(2), the side reaction was less likely to occur. Using CH(2)Cl(2) as solvent in coupling the unit, the target Abeta1-42 isopeptide was synthesized with almost no major side reaction.


