GlipizideCAS# 29094-61-9 |
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 29094-61-9 | SDF | Download SDF |
| PubChem ID | 3478 | Appearance | Powder |
| Formula | C21H27N5O4S | M.Wt | 445.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (112.22 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
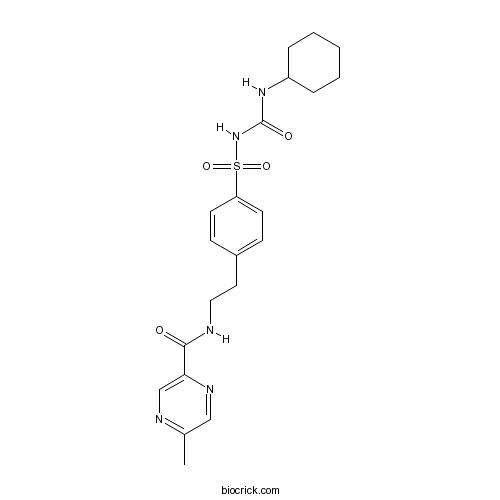
| Chemical Name | N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide | ||
| SMILES | CC1=NC=C(N=C1)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3 | ||
| Standard InChIKey | ZJJXGWJIGJFDTL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H27N5O4S/c1-15-13-24-19(14-23-15)20(27)22-12-11-16-7-9-18(10-8-16)31(29,30)26-21(28)25-17-5-3-2-4-6-17/h7-10,13-14,17H,2-6,11-12H2,1H3,(H,22,27)(H2,25,26,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Glipizide Dilution Calculator

Glipizide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2445 mL | 11.2223 mL | 22.4447 mL | 44.8893 mL | 56.1117 mL |
| 5 mM | 0.4489 mL | 2.2445 mL | 4.4889 mL | 8.9779 mL | 11.2223 mL |
| 10 mM | 0.2244 mL | 1.1222 mL | 2.2445 mL | 4.4889 mL | 5.6112 mL |
| 50 mM | 0.0449 mL | 0.2244 mL | 0.4489 mL | 0.8978 mL | 1.1222 mL |
| 100 mM | 0.0224 mL | 0.1122 mL | 0.2244 mL | 0.4489 mL | 0.5611 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Glipizide is an ATP-dependent K+ channels blocker in pancreatic β cells and brain GABA containing neurons resulting in insulin release.
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
- Avosentan
Catalog No.:BCC1387
CAS No.:290815-26-8
- Eupteleasaponin I
Catalog No.:BCN7839
CAS No.:290809-29-9
- 5-Hydroxy-3',4',7-trimethoxyflavone
Catalog No.:BCN5194
CAS No.:29080-58-8
- Pachymic acid
Catalog No.:BCN6347
CAS No.:29070-92-6
- Hexanorcucurbitacin D
Catalog No.:BCN7875
CAS No.:29065-05-2
- Matricin
Catalog No.:BCC8209
CAS No.:29041-35-8
- Bis(2-carboxyethyl)isocyanurate
Catalog No.:BCC8880
CAS No.:2904-40-7
- BMS 299897
Catalog No.:BCC2340
CAS No.:290315-45-6
- Glucosamine sulfate
Catalog No.:BCN5981
CAS No.:29031-19-4
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- 3-Epiglochidiol
Catalog No.:BCN5193
CAS No.:29028-10-2
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
- [Ala11,22,28]VIP
Catalog No.:BCC5754
CAS No.:291524-04-4
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- Adenine HCl
Catalog No.:BCC4453
CAS No.:2922-28-3
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
Glipizide blocks renal interstitial fibrosis by inhibiting AKT signaling pathway.[Pubmed:28272693]
Eur Rev Med Pharmacol Sci. 2017 Feb;21(4):867-872.
OBJECTIVE: Diabetes affects the renal function at a certain stage. Oral medication Glipizide plays a hypoglycemic effect mainly through releasing insulin, while more insulin is derived from islet beta cells. It is still controversy whether antidiabetics. This study mainly intends to investigate the role of Glipizide in inhibiting renal interstitial fibrosis. MATERIALS AND METHODS: A total of 93 SD rats were purchased from Guangdong animal monitoring and established unilateral ureteral obstruction (UUO) model to simulate renal interstitial fibrosis. Forty rats in the experimental group received Glipizide intraperitoneal injection for a week at 30 days after modeling, while another 40 rats in the control group received a normal saline injection. The last 10 rats were treated as blank group. Hematoxylin and eosin (HE) staining was applied to test renal interstitial fibrosis. Immunohistochemistry was used to detect fibronectin expression in glomerular and renal tubules. AKT signaling pathway related factors expression was measured by Western blot to determine AKT signal activation. RESULTS: HE staining showed that the entire kidney cytoplasm red dye becomes shallow, renal medulla gradually disappears, renal tubular epithelial cells enlarge, vacuoles degeneration, renal tubule and collecting tube expansion, inflammatory cells infiltration after UUO modeling. Glipizide treatment decreased dilated renal tubule number, improved glomerulus integrity, and reduced inflammatory infiltration. Fibronectin level in the experimental group was significantly lower than that in control (p<0.05). Western blot revealed that p-AKT expression downregulated after Glipizide treatment. CONCLUSIONS: Glipizide blocks renal interstitial fibrosis by inhibiting AKT signaling pathway.
Simultaneous determination of glipizide and its four hydroxylated metabolites in human urine using LC-MS/MS and its application in urinary phenotype study.[Pubmed:28284082]
J Pharm Biomed Anal. 2017 May 30;139:179-186.
Cytochrome P450 (CYP) 2C9 and CYP2C19 genetic mutant could influence the plasma concentration of Glipizide in human subjects, which refers to Glipizide safety and adverse effects in clinic practice. A further study to investigate the relationship of the concentrations between Glipizide and its metabolites in human with different CYP mutants was valuable. We firstly develop a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for simultaneous quantification of Glipizide and its hydroxylated metabolites in human urine. After simple protein precipitation with methanol including 4'-OH-tolbutamide and gliclazide (both are internal standards), the analytes were chromatographed on a reversed-phased column with a mobile phase of 0.1% formic acid in acetonitrile and 0.1% formic acid in water by a gradient elution. The ion transitions of the precursor to the product ion were principally protonated ions [M+H](+) at m/z 446.4-->m/z 321.1 for Glipizide, m/z 462.2-->m/z 321.1 for the four hydroxylated forms of Glipizide, m/z 287.2-->m/z 188.0 for 4'-OH-tolbutamide, and m/z 324.1-->m/z 127.1 for gliclazide. The method was linear over a concentration range of 0.02-20.0ng/mL. The intraday and inter-day variances were less than 9.9%, and accuracy was within +/-6.8%. The method was successfully applied to the urinary phenotyping study in volunteers after a single oral administration of 5-mg Glipizide tablet, and two new hydroxycyclohexyl metabolites of Glipizide (OH-gp), 4-cis-OH-gp and 3-trans-OH-gp, were found in this study.
Stable Co-crystals of Glipizide with Enhanced Dissolution Profiles: Preparation and Characterization.[Pubmed:28176212]
AAPS PharmSciTech. 2017 Oct;18(7):2454-2465.
Present study deciphers preparation of co-crystals of lipophilic Glipizide by using four different acids, oxalic, malonic, stearic, and benzoic acids, in order to achieve enhanced solubility and dissolution along with stability. All co-crystals were prepared by dissolving drug and individual acids in the ratio of 1:0.5 in acetonitrile at 60-70 degrees C for 15 min, followed by cooling at room temperature for 24 h. FT-IR spectroscopy revealed no molecular interaction between acids and drug as the internal structure and their geometric configurations remain unchanged. Differential scanning calorimetry revealed closer melting points of raw Glipizide and its co-crystals, which speculates absence of difference in crystallinity as well as intermolecular bonding of the co-crystals and drug. PXRD further revealed that all the co-crystals were having similar crystallinity as that of raw Glipizide except Glipizide-malonic acid co-crystals. This minor difference in the relative intensities of some of the diffraction peaks could be attributed to the crystal habit or crystal size modification. SEM revealed difference in the crystal morphology for all the co-crystals. Micromeritic, solubility, dissolution, and stability data revealed that among all the prepared co-crystals, Glipizide-stearic acid co-crystals were found superior. Hence, it was concluded that Glipizide-stearic acid co-crystals could offer an improved drug design strategy to overcome dissolution and bioavailability related challenges associated with lipophilic Glipizide.
Supersaturated controlled release matrix using amorphous dispersions of glipizide.[Pubmed:27492020]
Int J Pharm. 2016 Sep 25;511(2):957-68.
Spray dried dispersions (SDDs) of Glipizide, a BCS Class II model drug, were prepared using various grades of hydroxypropyl methylcellulose acetate succinate (HPMCAS) and copovidone S-630 as carriers. The SDDs appeared as a single amorphous phase with up to 60% drug loading level as revealed by X-ray powder diffraction (XRPD), modulated differential scanning calorimetry (mDSC) and scanning electron microscopy (SEM). Supersaturated micro-dissolution testing of various SDDs in fasted state simulated intestinal fluid showed prolonged supersaturation state (up to 180min) with solubility increases of 5.2-13.9 fold relative to crystalline drug under similar conditions. Solubility and stability characteristics of the most desirable SDDs in terms of relative dissolution AUCs (AUC(SDD)/AUC(crystalline)) and supersaturated concentration ratios (C180/Cmax) were determined. Results show that HPMCAS-based SDDs achieve a higher degree of supersaturation compared to Copovidone S-630 and that SDDs comprising HPMCAS-M and HPMCAS-H maintained stable supersaturated concentration. Dissolution data showed that SDD-loaded CR tablets provide stable supersaturated concentration within the hydrated matrix with increased rate and extent of drug dissolution over 24h. Co-existence of HPMCAS and HPMC within the hydrating matrix showed strong suppression of drug crystallization and allowed achievement of zero-order and slow-first order release kinetics.


