Glyasperin FCAS# 145382-61-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 145382-61-2 | SDF | Download SDF |
| PubChem ID | 392442 | Appearance | Powder |
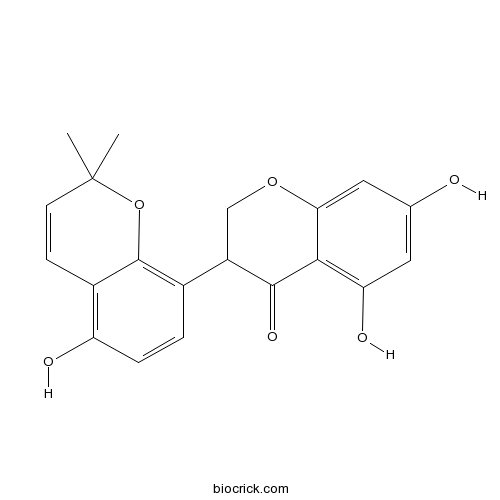
| Formula | C20H18O6 | M.Wt | 354.35 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5,7-dihydroxy-3-(5-hydroxy-2,2-dimethylchromen-8-yl)-2,3-dihydrochromen-4-one | ||
| SMILES | CC1(C=CC2=C(C=CC(=C2O1)C3COC4=CC(=CC(=C4C3=O)O)O)O)C | ||
| Standard InChIKey | CFCUNFSHJIQKLS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H18O6/c1-20(2)6-5-12-14(22)4-3-11(19(12)26-20)13-9-25-16-8-10(21)7-15(23)17(16)18(13)24/h3-8,13,21-23H,9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Glyasperin F has antinociceptive, and anti-inflammatory effects, it could inhibit NO production in RAW 264.7 macrophages to some extent. Glyasperin F displayed cytotoxic effects against the four tested cancer cell lines with IC50values below 85 μM. |
| Targets | NO |
| In vitro | Study on active constituents from Glycyrrhizae Radix et Rhizoma against NO production in LPS-induced RAW264.7 macrophages.[Reference: WebLink]Chinese Traditional and Herbal Drugs, 2016.To study the anti-inflammatory constituents in Glycyrrhizae Radix et Rhizoma. Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells.[Reference: WebLink]Bmc Complementary & Alternative Medicine, 2018, 18(1):36.Despite the remarkable progress in cancer therapy in recent years, this disease still remains a serious public health concern. The use of natural products has been and continues to be one of the most effective ways to fight malignancies. The cytotoxicity of 14 compounds from African medicinal plants was evaluated in four human carcinoma cell lines and normal fibroblasts.
The tested samples included: β-spinasterol (1), friedelanone (2), 16β-hydroxylupeol (3), β-amyrin acetate (4), lupeol acetate (5), sequoyitol (6), rhamnitrin (7), europetin 3-O-rhamnoside (8), thonningiol (9), Glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14). Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells.[Reference: WebLink]Bmc Complementary & Alternative Medicine, 2018, 18(1):36.Despite the remarkable progress in cancer therapy in recent years, this disease still remains a serious public health concern. The use of natural products has been and continues to be one of the most effective ways to fight malignancies. The cytotoxicity of 14 compounds from African medicinal plants was evaluated in four human carcinoma cell lines and normal fibroblasts.
The tested samples included: β-spinasterol (1), friedelanone (2), 16β-hydroxylupeol (3), β-amyrin acetate (4), lupeol acetate (5), sequoyitol (6), rhamnitrin (7), europetin 3-O-rhamnoside (8), thonningiol (9), Glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14). |
| In vivo | Antinociceptive effect of glyasperin F isolated from Glycyrrhiza inflata in mice.[Reference: WebLink]Journal of the Korean Society for Applied Biological Chemistry, 2013, 56(5):541–545.Antinociceptive effect of Glyasperin F isolated from Glycyrrhiza inflata extract (GIE) in ICR mice was studied.
|
| Structure Identification | Chinese Traditional and Herbal Drugs, 2010, 12(6):505-515.Chemical constituents in stem bark of Morus cathayana.[Reference: WebLink]To study chemical constituents in the stem bark of Morus cathayana. |

Glyasperin F Dilution Calculator

Glyasperin F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8221 mL | 14.1103 mL | 28.2207 mL | 56.4414 mL | 70.5517 mL |
| 5 mM | 0.5644 mL | 2.8221 mL | 5.6441 mL | 11.2883 mL | 14.1103 mL |
| 10 mM | 0.2822 mL | 1.411 mL | 2.8221 mL | 5.6441 mL | 7.0552 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5644 mL | 1.1288 mL | 1.411 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5644 mL | 0.7055 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bacopaside V
Catalog No.:BCN8629
CAS No.:620592-16-7
- Saikosaponin G
Catalog No.:BCN8628
CAS No.:99365-19-2
- 17-Hydroxyisolathyrol
Catalog No.:BCN8627
CAS No.:93551-00-9
- Leonloside D
Catalog No.:BCN8626
CAS No.:20830-84-6
- Lucidenic acid E
Catalog No.:BCN8625
CAS No.:98665-17-9
- Seconeokadsuranic acid A
Catalog No.:BCN8624
CAS No.:124817-74-9
- Laetanine
Catalog No.:BCN8623
CAS No.:72361-67-2
- Sibirioside A
Catalog No.:BCN8622
CAS No.:173046-19-0
- Periplocoside M
Catalog No.:BCN8621
CAS No.:116782-73-1
- Onjisaponin Z
Catalog No.:BCN8620
CAS No.:1078708-72-1
- Sargentol
Catalog No.:BCN8619
CAS No.:623928-18-7
- 3,4,4a,9,10,10a-heexahydro-8-hydroxy-1-(hydroxymethyl)-1,4a-dimethyl-7-(1-methylethyl)-phenanthrene-2(1H)-one
Catalog No.:BCN8618
CAS No.:262599-12-2
- 2-Amino-N-(2-bromophenyl)benzamide
Catalog No.:BCN8631
CAS No.:34489-85-5
- Saikosaponin E
Catalog No.:BCN8632
CAS No.:64340-44-9
- 2,3,4,5-Tetracaffeoyl-D-glucaric acid
Catalog No.:BCN8633
CAS No.:1419478-52-6
- Kushenol S
Catalog No.:BCN8634
CAS No.:254886-72-1
- Dihydrolicoisoflavone
Catalog No.:BCN8635
CAS No.:164163-92-2
- (Z)-Aldosecologanin
Catalog No.:BCN8636
CAS No.:82474-97-3
- Veratrosine
Catalog No.:BCN8637
CAS No.:475-00-3
- Bruceoside A
Catalog No.:BCN8638
CAS No.:63306-30-9
- Glabrone
Catalog No.:BCN8639
CAS No.:60008-02-8
- Hypaconine
Catalog No.:BCN8640
CAS No.:63238-68-6
- Lancifodilactone C
Catalog No.:BCN8641
CAS No.:663176-26-9
- Myrrhone
Catalog No.:BCN8642
CAS No.:183551-83-9
Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells.[Pubmed:29378558]
BMC Complement Altern Med. 2018 Jan 30;18(1):36.
BACKGROUND: Despite the remarkable progress in cancer therapy in recent years, this disease still remains a serious public health concern. The use of natural products has been and continues to be one of the most effective ways to fight malignancies. The cytotoxicity of 14 compounds from African medicinal plants was evaluated in four human carcinoma cell lines and normal fibroblasts. The tested samples included: beta-spinasterol (1), friedelanone (2), 16beta-hydroxylupeol (3), beta-amyrin acetate (4), lupeol acetate (5), sequoyitol (6), rhamnitrin (7), europetin 3-O-rhamnoside (8), thonningiol (9), Glyasperin F (10), seputhecarpan B (11), seputhecarpan C (12), seputhecarpan D (13) and rheediaxanthone A (14). METHODS: The neutral red uptake (NR) assay was used to evaluate the cytotoxicity of samples; caspase-Glo assay, flow cytometry for cell cycle analysis and mitochondrial membrane potential (MMP) as well as spectrophotometry to measure levels of reactive oxygen species (ROS) were performed to detect the mode of action of compounds 9 and 13 in MCF-7 breast adenocarcinoma cells. RESULTS: Compounds 3, 9-13 displayed cytotoxic effects against the four tested cancer cell lines with IC50 values below 85 muM. Compounds 9 and 13 had IC50 values below 10 muM in 4/4 and 3/4 tested cell lines respectively. The IC50 values varied from 0.36 muM (against MCF7 cells) to 5.65 muM (towards colon carcinoma DLD-1 cells) for 9, from 9.78 muM (against MCF7 cells) to 67.68 muM (against HepG2 cells) for 13 and 0.18 muM (towards HepG2 cells) to 72 muM (towards Caco-2 cells) for the reference drug, doxorubicin. Compounds 9 and 13 induced cell cycle arrest in Go/G1 whilst doxorubicin induced arrest in G2/M. The two molecules (9 and 13) also induced apoptosis in MCF-7 cells through activation of caspases 3/7 and 9 as well as enhanced ROS production. CONCLUSION: Compounds 9 and 13 are good cytotoxic phytochemicals that should be explored more in future to develop a cytotoxic drug to fight human carcinoma.
Two new pterocarpans and a new pyrone derivative with cytotoxic activities from Ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae): revised NMR assignment of mundulea lactone.[Pubmed:28316643]
Chem Cent J. 2016 Oct 5;10:58.
BACKGROUND: Ptycholobium is a genus related to Tephrosia which comprises only three species. Compared to Tephrosia, which has been phytochemically and pharmacologically studied, Ptycholobium species have only few or no reports on their chemical constituents. Moreover, no studies on the cytotoxic activities of its secondary metabolites have been previously documented. RESULTS: From the non polar fractions of the roots bark of Ptycholobium contortum (syn Tephrosia contorta), two new pterocarpans: seputhecarpan C 1 and seputhecarpan D 2 and a new pyrone derivative, ptycholopyrone A 3 were isolated. Alongside, five known compounds identified as 3-alpha,alpha-dimethylallyl-4-methoxy-6-styryl-alpha-pyrone or mundulea lactone 4, Glyasperin F 5, seputhecarpan A 6, seputheisoflavone 7 and 5-O-methyl-myo-inositol or sequoyitol 8 were also obtained. Their structures were established by the mean means of spectroscopic data in conjunction to those reported in literature. The NMR assignment of the major compound mundulea lactone 4 is revised in this paper. In addition, the cytotoxicity of the isolated metabolites was evaluated on two lung cancer cell lines A549 and SPC212. 8 was not active while compounds 1, 2, 4-7 displayed antiproliferative effects against the two carcinoma cell lines with IC50 values below 75 microM. IC50 values below 10 microM were obtained for 4, 6 and 7 on SPC212 cells. CONCLUSION: Based on the obtained results, Ptycholobium contortum turns to be a rich source of phenolic metabolites among them some bearing prenyl moieties. This study reports for the first time the isolation of pyrone derivatives 3 and 4 from Ptycholobium genus. The cytotoxicity observed for the isolate is also reported for the first time and shows that 4, 6 and 7 could be chemically explored in order to develop a hit candidate against lung cancer. Graphical abstractTwo new pterocarpans and a new pyrone derivative with cytotoxic activities from ptycholobium contortum (N.E.Br.) Brummitt (Leguminosae): revised NMR assignment of mundulea lactone.


