HC-030031TRPA1 channel blocker,potent and selective CAS# 349085-38-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 349085-38-7 | SDF | Download SDF |
| PubChem ID | 1150897 | Appearance | Powder |
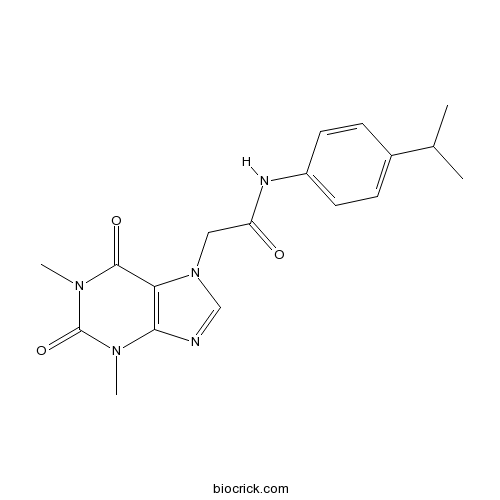
| Formula | C18H21N5O3 | M.Wt | 355.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 25 mg/mL (70.35 mM; Need ultrasonic) | ||
| Chemical Name | 2-(1,3-dimethyl-2,6-dioxopurin-7-yl)-N-(4-propan-2-ylphenyl)acetamide | ||
| SMILES | CC(C)C1=CC=C(C=C1)NC(=O)CN2C=NC3=C2C(=O)N(C(=O)N3C)C | ||
| Standard InChIKey | HEQDZPHDVAOBLN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H21N5O3/c1-11(2)12-5-7-13(8-6-12)20-14(24)9-23-10-19-16-15(23)17(25)22(4)18(26)21(16)3/h5-8,10-11H,9H2,1-4H3,(H,20,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective TRPA1 channel blocker that antagonizes AITC- and formalin-evoked calcium influx (IC50 values are 6.2 and 5.3 μM respectively). Does not block currents mediated by TRPV1, TRPV3, TRPV4, hERG or NaV1.2 channels. Inhibits AITC- and formalin-induced flinching in vivo. |

HC-030031 Dilution Calculator

HC-030031 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8138 mL | 14.0691 mL | 28.1381 mL | 56.2762 mL | 70.3453 mL |
| 5 mM | 0.5628 mL | 2.8138 mL | 5.6276 mL | 11.2552 mL | 14.0691 mL |
| 10 mM | 0.2814 mL | 1.4069 mL | 2.8138 mL | 5.6276 mL | 7.0345 mL |
| 50 mM | 0.0563 mL | 0.2814 mL | 0.5628 mL | 1.1255 mL | 1.4069 mL |
| 100 mM | 0.0281 mL | 0.1407 mL | 0.2814 mL | 0.5628 mL | 0.7035 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
HC-030031 is a selective antagonist of transient receptor potential ankyrin subfamily, member 1(TRPA1) with IC50 value of 4.9μM [1].
TRP channels are found to take participate in the response of chemical stimuli and in mechanoreception. TRPA1 is the sole mammalian member of TRPA subfamily. As an inhibitor of TRPA1, HC-030031 can be used as a tool to study the role of this channel in pain perception. In the FLIPR calcium-influx assay, HC-030031 blocks cinnamaldehyde- and AITC- induced activation of TRPA1 with IC50 values of 4.9μM and 7.5μM, respectively. HC-030031 is selective against TRPA1. It shows no significant inhibitory activity against many other enzymes, receptors and transporters. It also has no effect on the activation of TRPV1, TRPV3 and TRPV4. In vivo, HC-030031 significantly reduces the hindpaw lifting induced by AITC as well as mechanical hypersensitivity induced by CFA in rats. Besides that, the administration of HC-030031(300mg/kg) can markedly attenuate the hypersensitivity (41%) induced by spinal nerve ligation [1].
References:
[1] Eid S R, Crown E D, Moore E L, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory-and neuropathy-induced mechanical hypersensitivity. Mol Pain, 2008, 4(48): 1-10.
- Vineridine
Catalog No.:BCN5286
CAS No.:3489-06-3
- UK 383367
Catalog No.:BCC2308
CAS No.:348622-88-8
- Palmatine
Catalog No.:BCN5285
CAS No.:3486-67-7
- Coptisine
Catalog No.:BCN6320
CAS No.:3486-66-6
- Optovin
Catalog No.:BCC6323
CAS No.:348575-88-2
- Methyl 3-methoxyacrylate
Catalog No.:BCN2258
CAS No.:34846-90-7
- 12-Hydroxyabietic acid
Catalog No.:BCN5284
CAS No.:3484-61-5
- Metiamide
Catalog No.:BCC1742
CAS No.:34839-70-8
- Bz-Tyr-Oet
Catalog No.:BCC3122
CAS No.:3483-82-7
- DL-Dithiothreitol
Catalog No.:BCC7586
CAS No.:3483-12-3
- 4'-Demethyleucomin
Catalog No.:BCN5283
CAS No.:34818-83-2
- Marilactone
Catalog No.:BCN7363
CAS No.:34818-17-2
- GSK 137647
Catalog No.:BCC8045
CAS No.:349085-82-1
- JX 401
Catalog No.:BCC7443
CAS No.:349087-34-9
- Cryptomeridiol 11-rhamnoside
Catalog No.:BCN4648
CAS No.:349112-30-7
- S-Adenosyl-L-Methionine iodide salt
Catalog No.:BCN2232
CAS No.:3493-13-8
- Alda 1
Catalog No.:BCC6096
CAS No.:349438-38-6
- Methyl dodonate A acetate
Catalog No.:BCN4647
CAS No.:349487-98-5
- Methyl dodonate A
Catalog No.:BCN4646
CAS No.:349534-70-9
- Dodonolide
Catalog No.:BCN4645
CAS No.:349534-73-2
- 3'',4''-Di-O-acetyl-2'',6''-di-O-p-coumaroylastragalin
Catalog No.:BCN6843
CAS No.:349545-02-4
- Z-Val-OSu
Catalog No.:BCC2733
CAS No.:3496-11-5
- Kushenol F
Catalog No.:BCN3798
CAS No.:34981-24-3
- Kuraridine
Catalog No.:BCN2988
CAS No.:34981-25-4
Blockade of TRPA1 with HC-030031 attenuates visceral nociception by a mechanism independent of inflammatory resident cells, nitric oxide and the opioid system.[Pubmed:22689151]
Eur J Pain. 2013 Feb;17(2):223-33.
BACKGROUND: Some studies have shown a somatic nociceptive response due to the activation of transient receptor potential A1 channels (TRPA1), which is modulated by the TRPA1 antagonist HC-030031. However, a few studies report the role of TRPA1 in visceral pain. Therefore, we investigated the participation of TRPA1 in visceral nociception and the involvement of nitric oxide, the opioid system and resident cells in the modulation of these channels. METHODS: Mice were treated with vehicle or HC-030031 (18.75-300 mg/kg) before ifosfamide (400 mg/kg), 0.75% mustard oil (50 muL/colon), acetic acid 0.6% (10 mL/kg), zymosan (1 mg/cavity) or misoprostol (1 mug/cavity) injection. Visceral nociception was assessed through the electronic von Frey test or the writhing response. Ifosfamide-administered mice were euthanized for bladder analysis. The involvement of nitric oxide and the opioid system were investigated in mice injected with ifosfamide and mustard oil, respectively. The participation of resident peritoneal cells in acetic acid-, zymosan- or misoprostol-induced nociception was also evaluated. RESULTS: HC-030031 failed to protect animals against ifosfamide-induced bladder injury (p > 0.05). However, a marked antinociceptive effect against ifosfamide, mustard oil, acetic acid, zymosan and misoprostol was observed (p < 0.05). Neither L-arginine (600 mg/kg) nor naloxone (2 mg/kg) could reverse the antinociceptive effect of HC-030031. The reduction of the peritoneal cell population inhibited the acetic acid and zymosan-related writhes without interfering with the misoprostol effect. CONCLUSIONS: Our findings suggest that the blockade of TRPA1 attenuates visceral nociception by a mechanism independent of the modulation of resident cells, nitric oxide and opioid pathways.
Structural basis of TRPA1 inhibition by HC-030031 utilizing species-specific differences.[Pubmed:27874100]
Sci Rep. 2016 Nov 22;6:37460.
Pain is a harmful sensation that arises from noxious stimuli. Transient receptor potential ankyrin 1 (TRPA1) is one target for studying pain mechanisms. TRPA1 is activated by various stimuli such as noxious cold, pungent natural products and environmental irritants. Since TRPA1 is an attractive target for pain therapy, a few TRPA1 antagonists have been developed and some function as analgesic agents. The responses of TRPA1 to agonists and antagonists vary among species and these species differences have been utilized to identify the structural basis of activation and inhibition mechanisms. The TRPA1 antagonist HC-030031 (HC) failed to inhibit frog TRPA1 (fTRPA1) and zebrafish TRPA1 activity induced by cinnamaldehyde (CA), but did inhibit human TRPA1 (hTRPA1) in a heterologous expression system. Chimeric studies between fTRPA1 and hTRPA1, as well as analyses using point mutants, revealed that a single amino acid residue (N855 in hTRPA1) significantly contributes to the inhibitory action of HC. Moreover, the N855 residue and the C-terminus region exhibited synergistic effects on the inhibition by HC. Molecular dynamics simulation suggested that HC stably binds to hTRPA1-N855. These findings provide novel insights into the structure-function relationship of TRPA1 and could lead to the development of more effective analgesics targeted to TRPA1.
HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity.[Pubmed:18954467]
Mol Pain. 2008 Oct 27;4:48.
BACKGROUND: Safe and effective treatment for chronic inflammatory and neuropathic pain remains a key unmet medical need for many patients. The recent discovery and description of the transient receptor potential family of receptors including TRPV1 and TRPA1 has provided a number of potential new therapeutic targets for treating chronic pain. Recent reports have suggested that TRPA1 may play an important role in acute formalin and CFA induced pain. The current study was designed to further explore the therapeutic potential of pharmacological TRPA1 antagonism to treat inflammatory and neuropathic pain. RESULTS: The in vitro potencies of HC-030031 versus cinnamaldehyde or allyl isothiocyanate (AITC or Mustard oil)-induced TRPA1 activation were 4.9 +/- 0.1 and 7.5 +/- 0.2 microM respectively (IC50). These findings were similar to the previously reported IC50 of 6.2 microM against AITC activation of TRPA1 1. In the rat, oral administration of HC-030031 reduced AITC-induced nocifensive behaviors at a dose of 100 mg/kg. Moreover, oral HC-030031 (100 mg/kg) significantly reversed mechanical hypersensitivity in the more chronic models of Complete Freunds Adjuvant (CFA)-induced inflammatory pain and the spinal nerve ligation model of neuropathic pain. CONCLUSION: Using oral administration of the selective TRPA1 antagonist HC-030031, our results demonstrated that TRPA1 plays an important role in the mechanisms responsible for mechanical hypersensitivity observed in inflammatory and neuropathic pain models. These findings suggested that TRPA1 antagonism may be a suitable new approach for the development of a potent and selective therapeutic agent to treat both inflammatory and neuropathic pain.
Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1).[Pubmed:18000030]
Mol Pharmacol. 2008 Feb;73(2):274-81.
Inflammation contributes to pain hypersensitivity through multiple mechanisms. Among the most well characterized of these is the sensitization of primary nociceptive neurons by arachidonic acid metabolites such as prostaglandins through G protein-coupled receptors. However, in light of the recent discovery that the nociceptor-specific ion channel transient receptor potential A1 (TRPA1) can be activated by exogenous electrophilic irritants through direct covalent modification, we reasoned that electrophilic carbon-containing A- and J-series prostaglandins, metabolites of prostaglandins (PG) E(2) and D(2), respectively, would excite nociceptive neurons through direct activation of TRPA1. Consistent with this prediction, the PGD(2) metabolite 15-deoxy-Delta(12,14)-prostaglandin J(2) (15dPGJ(2)) activated heterologously expressed human TRPA1 (hTRPA1-HEK), as well as a subset of chemosensitive mouse trigeminal neurons. The effects of 15dPGJ(2) on neurons were blocked by both the nonselective TRP channel blocker ruthenium red and the TRPA1 inhibitor (HC-030031), but unaffected by the TRPV1 blocker iodo-resiniferatoxin. In whole-cell patch-clamp studies on hTRPA1-HEK cells, 15dPGJ(2) evoked currents similar to equimolar allyl isothiocyanate (AITC) in the nominal absence of calcium, suggesting a direct mechanism of activation. Consistent with the hypothesis that TRPA1 activation required reactive electrophilic moieties, A- and J-series prostaglandins, and the isoprostane 8-iso-prostaglandin A(2)-evoked calcium influx in hTRPA1-HEK cells with similar potency and efficacy. It is noteworthy that this effect was not mimicked by their nonelectrophilic precursors, PGE(2) and PGD(2), or PGB(2), which differs from PGA(2) only in that its electrophilic carbon is rendered unreactive through steric hindrance. Taken together, these data suggest a novel mechanism through which reactive prostanoids may activate nociceptive neurons independent of prostaglandin receptors.


