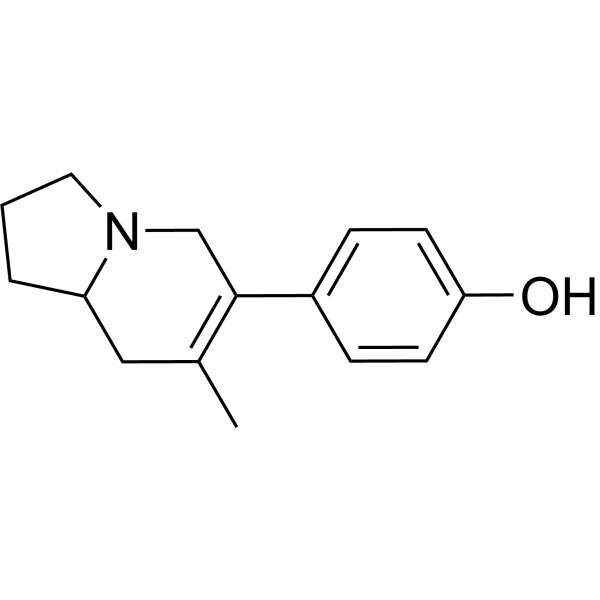
IpalbidineCAS# 26294-41-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 26294-41-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C15H19NO | M.Wt | 229.32 |
| Type of Compound | Piperidines | Storage | Desiccate at -20°C |
| Synonyms | (±)-Ipalbidine,(±)-Ipalbidin | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ipalbidine Dilution Calculator

Ipalbidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3607 mL | 21.8036 mL | 43.6072 mL | 87.2144 mL | 109.018 mL |
| 5 mM | 0.8721 mL | 4.3607 mL | 8.7214 mL | 17.4429 mL | 21.8036 mL |
| 10 mM | 0.4361 mL | 2.1804 mL | 4.3607 mL | 8.7214 mL | 10.9018 mL |
| 50 mM | 0.0872 mL | 0.4361 mL | 0.8721 mL | 1.7443 mL | 2.1804 mL |
| 100 mM | 0.0436 mL | 0.218 mL | 0.4361 mL | 0.8721 mL | 1.0902 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Piperkadsin A
Catalog No.:BCX1813
CAS No.:895543-36-9
- Heilaohuguosu G
Catalog No.:BCX1812
CAS No.:2763689-78-5
- Kadsuphilin A
Catalog No.:BCX1811
CAS No.:887770-02-7
- Isolaureline
Catalog No.:BCX1810
CAS No.:475-84-3
- Schisantherin S
Catalog No.:BCX1809
CAS No.:2230512-49-7
- 2,5,6-Trihydroxy-3,4-methoxy-9,10-dihydrophenanthrene
Catalog No.:BCX1808
CAS No.:1810733-76-6
- (2R,3R,5S)-2-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5-methoxy-3-methyl-5-( 2-propenyl) -6(2H)-benzofuran...
Catalog No.:BCX1807
CAS No.:155551-59-0
- Kadsurenin B
Catalog No.:BCX1806
CAS No.:145701-13-9
- Siderin
Catalog No.:BCX1805
CAS No.:53377-54-1
- Jervinone
Catalog No.:BCX1804
CAS No.:469-60-3
- Soladulcoside A
Catalog No.:BCX1803
CAS No.:137031-53-9
- Kaempferol 3-O-(6-O-(E)-caffeoyl-β-D-glucopyranosyl-(1→3)-α-L-rhamnopyranoside) 7-O-α-L-rhamnopyrano...
Catalog No.:BCX1802
CAS No.:1459767-45-3
- Ipalbine
Catalog No.:BCX1815
CAS No.:23544-46-9
- Lysimachigenoside C
Catalog No.:BCX1816
CAS No.:952304-73-3
- Foenumoside B
Catalog No.:BCX1817
CAS No.:877661-00-2
- Changnanic acid
Catalog No.:BCX1818
CAS No.:136040-44-3
- Manwuweizic acid
Catalog No.:BCX1819
CAS No.:116963-87-2
- Grandivine A
Catalog No.:BCX1820
CAS No.:200574-22-7
- (7S,8S,1′S)-3,4,1′-Trimethoxy-1′,6′-dihydro-7,4′-epoxy-8,3′-neoligna-8′-en-6′-one
Catalog No.:BCX1821
CAS No.:61240-30-0
- Puberulin A
Catalog No.:BCX1822
CAS No.:164268-04-6
- (S)-(+)-Imperanene
Catalog No.:BCX1823
CAS No.:163634-08-0
- 5-Hydroxy-2-(2′-hydroxy-2-phenylethyl)chromone
Catalog No.:BCX1824
CAS No.:357637-15-1
- Isoguaiacin
Catalog No.:BCX1825
CAS No.:78341-26-1
- Rubriflorin B
Catalog No.:BCX1826
CAS No.:835875-96-2
Improved Total Synthesis of Indolizidine and Quinolizidine Alkaloids via Nickel-Catalyzed (4 + 2) Cycloaddition.[Pubmed:35759553]
J Org Chem. 2022 Jul 15;87(14):8871-8883.
A Ni-catalyzed (4 + 2) cycloaddition of bicyclic 3-azetidinones and alkynes was developed to access indolizidine and quinolizidine alkaloids. A key element was the development of a diazomethylation procedure that allows the efficient synthesis of bicyclic azetidinones from pyroglutamic and 6-oxopiperidine-2-carboxylic acid. A ligand screening led to improved regioselectivity and enantiopurity during the Ni-catalyzed (4 + 2) cycloaddition. This straightforward methodology was leveraged to synthesize (+)-Ipalbidine, (+)-septicine, (+)-seco-antofine, and (+)-7-methoxy-julandine.
Total Synthesis of Indolizidine Alkaloids via Nickel-Catalyzed (4 + 2) Cyclization.[Pubmed:31928010]
Org Lett. 2020 Feb 7;22(3):924-928.
A Ni-catalyzed (4 + 2) cycloaddition of alkynes and azetidinones toward piperidinones was used as key reaction in the enantioselective synthesis of naturally occurring indolizidine alkaloids. The reaction benefits from the use of an easily accessible azetidinone as an advanced and divergent intermediate to build the indolizidine core. This methodology has been applied in the total syntheses of (+)-septicine, (+)-Ipalbidine, and (+)-seco-antofine to illustrate the applicability of the general approach.
Simple Indolizidine and Quinolizidine Alkaloids.[Pubmed:26777309]
Alkaloids Chem Biol. 2016;75:1-498.
This review of simple indolizidine and quinolizidine alkaloids (i.e., those in which the parent bicyclic systems are in general not embedded in polycyclic arrays) is an update of the previous coverage in Volume 55 of this series (2001). The present survey covers the literature from mid-1999 to the end of 2013; and in addition to aspects of the isolation, characterization, and biological activity of the alkaloids, much emphasis is placed on their total synthesis. A brief introduction to the topic is followed by an overview of relevant alkaloids from fungal and microbial sources, among them slaframine, cyclizidine, Steptomyces metabolites, and the pantocins. The important iminosugar alkaloids lentiginosine, steviamine, swainsonine, castanospermine, and related hydroxyindolizidines are dealt with in the subsequent section. The fourth and fifth sections cover metabolites from terrestrial plants. Pertinent plant alkaloids bearing alkyl, functionalized alkyl or alkenyl substituents include dendroprimine, anibamine, simple alkaloids belonging to the genera Prosopis, Elaeocarpus, Lycopodium, and Poranthera, and bicyclic alkaloids of the lupin family. Plant alkaloids bearing aryl or heteroaryl substituents include Ipalbidine and analogs, secophenanthroindolizidine and secophenanthroquinolizidine alkaloids (among them septicine, julandine, and analogs), ficuseptine, lasubines, and other simple quinolizidines of the Lythraceae, the simple furyl-substituted Nuphar alkaloids, and a mixed quinolizidine-quinazoline alkaloid. The penultimate section of the review deals with the sizable group of simple indolizidine and quinolizidine alkaloids isolated from, or detected in, ants, mites, and terrestrial amphibians, and includes an overview of the "dietary hypothesis" for the origin of the amphibian metabolites. The final section surveys relevant alkaloids from marine sources, and includes clathryimines and analogs, stellettamides, the clavepictines and pictamine, and bis(quinolizidine) alkaloids.
Synthesis of (+)-Ipalbidine Based on 6-exo-trig Radical Cyclization of a beta-Amino Radical.[Pubmed:26402510]
J Org Chem. 2015 Oct 16;80(20):10294-8.
N-Boc (S)-proline was converted into (2S)-2-[(phenylselanyl)methyl]pyrrolidine, which was alkylated on nitrogen with 2-bromo-1-(4-methoxyphenyl)ethan-1-one. Reaction with vinyllithium, 6-exo-trig radical cyclization (Bu3SnH, AIBN, PhMe, 110 degrees C), dehydration (P2O5, H3PO4), and demethylation (BBr3) gave (+)-Ipalbidine with ee >99%.
Total syntheses of arylindolizidine alkaloids (+)-ipalbidine and (+)-antofine.[Pubmed:20704319]
J Org Chem. 2010 Sep 3;75(17):6019-22.
This paper presents the first application of two recently developed reactions to natural product synthesis. The first method involves a 6-endo-trig cyclization to prepare a versatile chiral enaminone building block. The second is a direct C-H arylation reaction. As a showcase for the utility of these methods, (+)-antofine and (+)-Ipalbidine were synthesized in only 8 steps and 24-26% overall yields.
Indolizidine and quinolizidine alkaloids.[Pubmed:15459758]
Nat Prod Rep. 2004 Oct;21(5):625-49.
This review covers the isolation, structure determination, synthesis, chemical transformations and biological activity of indolizidine and quinolizidine alkaloids from microbial, plant and animal sources. Included in the review are the hydroxylated indolizidines lentiginosine, swainsonine, castanospermine and their analogues; alkaloids from animal sources, including ants, amphibians and beetles; Ipalbidine, phenanthroindolizidines and related alkaloids; Lycopodium alkaloids; lupine alkaloids; and alkaloids from bacterial and marine sources. The literature from July 2002 to June 2003 is reviewed, and 174 references are cited.
Indolizidine and quinolizidine alkaloids.[Pubmed:14620842]
Nat Prod Rep. 2003 Oct;20(5):458-75.
This review covers the isolation, structure determination, synthesis, chemical transformations and biological activity of indolizidine and quinolizidine alkaloids from microbial, plant and animal sources. Included in the review are slaframine; the hydroxylated indolizidines lentiginosine, swainsonine, castanospermine and their analogues; alkaloids from amphibians and marine sources; plumerinine; Ipalbidine, phenanthroindolizidines and related alkaloids; lasubine-II: and lupin alkaloids. The literature from July 2001 to June 2002 is reviewed, and 142 references are cited.
[Effect of norepinephrinergic system on ipalbidine analgesia].[Pubmed:9863250]
Yao Xue Xue Bao. 1996;31(11):806-11.
Ipalbidine (Ipa) is a photoactive alkaloid isolated from the seeds of ipomoea hardwickki Hemsl. The analgesic effects of Ipa were determined by rat tail flick method. A dose-dependent analgesic effect was found after s.c. or i.c.v. administration of Ipa, but no analgesia was observed after intrathecal injection, indicating that the analgesic effect of Ipa is central in origin, and it acts mainly on supraspinal substrate. The analgesic effect induced by Ipa (60 mg.kg-1, s.c.) was markedly reduced by reserpine (2 mg.kg-1, i.p.) given 24 h before Ipa, which was reversed by combined administration with i.c.v. norepinephrine (NE). In addition, Ipa-induced analgesia was significantly attenuated by electrolytic lesion of bilateral destruction of locus coeruleus, and combined administration with diethyldithiocarbamate (200 mg.kg-1, i.p.), phentolamine (10 mg.kg-1, i.p. or i.c.v.), and prazosin (3 mg.kg-1, s.c.). But no influence was observed on the analgesia of Ipa after administration of yohimbine (5 mg.kg-1, s.c.) or propranolol (10 mg.kg-1, i.p.). These results suggest that the analgesia caused by Ipa is closely related to the function of the central norepinephrinergic system, and probably mediated by indirectly acting on alpha 1 receptors, but not alpha 2 or beta receptors.


