LY2119620positive allosteric modulator of M2/M4 receptor CAS# 886047-22-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 886047-22-9 | SDF | Download SDF |
| PubChem ID | 57664406 | Appearance | Powder |
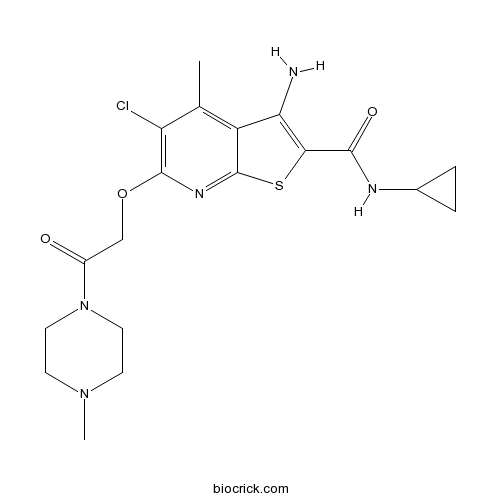
| Formula | C19H24ClN5O3S | M.Wt | 437.94 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 67.5 mg/mL (154.13 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 3-amino-5-chloro-N-cyclopropyl-4-methyl-6-[2-(4-methylpiperazin-1-yl)-2-oxoethoxy]thieno[2,3-b]pyridine-2-carboxamide | ||
| SMILES | CC1=C2C(=C(SC2=NC(=C1Cl)OCC(=O)N3CCN(CC3)C)C(=O)NC4CC4)N | ||
| Standard InChIKey | TYTGOXSAAQWLPJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H24ClN5O3S/c1-10-13-15(21)16(17(27)22-11-3-4-11)29-19(13)23-18(14(10)20)28-9-12(26)25-7-5-24(2)6-8-25/h11H,3-9,21H2,1-2H3,(H,22,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

LY2119620 Dilution Calculator

LY2119620 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2834 mL | 11.4171 mL | 22.8342 mL | 45.6684 mL | 57.0854 mL |
| 5 mM | 0.4567 mL | 2.2834 mL | 4.5668 mL | 9.1337 mL | 11.4171 mL |
| 10 mM | 0.2283 mL | 1.1417 mL | 2.2834 mL | 4.5668 mL | 5.7085 mL |
| 50 mM | 0.0457 mL | 0.2283 mL | 0.4567 mL | 0.9134 mL | 1.1417 mL |
| 100 mM | 0.0228 mL | 0.1142 mL | 0.2283 mL | 0.4567 mL | 0.5709 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2119620 is a selective positive allosteric modulator of M2/M4 receptor [1].
Muscarinic acetylcholine receptors (M1-M5) are G-protein coupled receptors regulating the action of the neurotransmitter ACh and play an important role in smooth muscle control, exocrine gland function, mood and cognition [1].
LY2119620 is a selective positive allosteric modulator of M2/M4 receptor. In [35S]GTPγS-binding experiments, LY2119620 exhibited a modest allosteric agonism of 23.2% and 16.8% at the M2 and M4 receptors, respectively. LY2119620 and ACh binding caused cooperativity factor α of 79.4 and 19.5 for the M4 receptor and the M2 receptor, respectively. The cooperativity between LY2119620 and orthosteric agonists (Iperoxo or Oxo-M) was also observed [1]. M2 receptor simultaneously bound to LY2119620 and iperoxo. LY2119620 exhibited mild negative cooperativity with the inverse agonist NMS and strong positive cooperativity with iperoxo [2]. LY2119620 significantly increased Bmax values without changes in Kd when cooperativity binding of [3H]LY2119620 with mAChR, suggesting a G protein-dependent process [3].
References:
[1]. Croy CH, Schober DA, Xiao H, et al. Characterization of the novel positive allosteric modulator, LY2119620, at the muscarinic M(2) and M(4) receptors. Mol Pharmacol, 2014, 86(1): 106-115.
Kruse AC, Ring AM, Manglik A, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature, 2013, 504(7478): 101-106.
Schober DA, Croy CH, Xiao H, et al. Development of a radioligand, [(3)H]LY2119620, to probe the human M(2) and M(4) muscarinic receptor allosteric binding sites. Mol Pharmacol, 2014, 86(1): 116-123.
- Isomexoticin
Catalog No.:BCN4434
CAS No.:88585-86-8
- Fmoc-ε-Acp-OH
Catalog No.:BCC3206
CAS No.:88574-06-5
- c-FMS inhibitor
Catalog No.:BCC1472
CAS No.:885704-21-2
- 5'-O-Acetyljuglanin
Catalog No.:BCN6846
CAS No.:885697-82-5
- CCT128930
Catalog No.:BCC3904
CAS No.:885499-61-6
- Minumicrolin
Catalog No.:BCN4433
CAS No.:88546-96-7
- HJC 0350
Catalog No.:BCC6302
CAS No.:885434-70-8
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- Kongensin A
Catalog No.:BCN4432
CAS No.:885315-96-8
- W-13 hydrochloride
Catalog No.:BCC6620
CAS No.:88519-57-7
- Rosamultin
Catalog No.:BCN7391
CAS No.:88515-58-6
- LY 2389575 hydrochloride
Catalog No.:BCC7985
CAS No.:885104-09-6
- Chrysin 7-O-beta-gentiobioside
Catalog No.:BCN2943
CAS No.:88640-89-5
- Cerebroside B
Catalog No.:BCN4435
CAS No.:88642-46-0
- Apogossypolone (ApoG2)
Catalog No.:BCC2237
CAS No.:886578-07-0
- 11-Hydroxy-sugiol
Catalog No.:BCN2510
CAS No.:88664-08-8
- 14-Deoxycoleon U
Catalog No.:BCN4796
CAS No.:88664-09-9
- 8-Epideoxyloganic acid
Catalog No.:BCN6576
CAS No.:88668-99-9
- Liranaftate
Catalog No.:BCC4672
CAS No.:88678-31-3
- Piperlotine C
Catalog No.:BCN6485
CAS No.:886989-88-4
- Neobritannilactone B
Catalog No.:BCN3510
CAS No.:886990-00-7
- Borneol 7-O-[beta-D-apiofuranosyl-(1->6)]-beta-D-glucopyranoside
Catalog No.:BCN7814
CAS No.:88700-35-0
- 1alpha-Hydroxytorilin
Catalog No.:BCN6648
CAS No.:887147-75-3
- Adynerigenin beta-neritrioside
Catalog No.:BCN4556
CAS No.:88721-09-9
Development of a radioligand, [(3)H]LY2119620, to probe the human M(2) and M(4) muscarinic receptor allosteric binding sites.[Pubmed:24807966]
Mol Pharmacol. 2014 Jul;86(1):116-23.
In this study, we characterized a muscarinic acetylcholine receptor (mAChR) potentiator, LY2119620 (3-amino-5-chloro-N-cyclopropyl-4-methyl-6-[2-(4-methylpiperazin-1-yl)-2-oxoethox y]thieno[2,3-b]pyridine-2-carboxamide) as a novel probe of the human M2 and M4 allosteric binding sites. Since the discovery of allosteric binding sites on G protein-coupled receptors, compounds targeting these novel sites have been starting to emerge. For example, LY2033298 (3-amino-5-chloro-6-methoxy-4-methyl-thieno(2,3-b)pyridine-2-carboxylic acid cyclopropylamid) and a derivative of this chemical scaffold, VU152100 (3-amino-N-(4-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide), bind to the human M4 mAChR allosteric pocket. In the current study, we characterized LY2119620, a compound similar in structure to LY2033298 and binds to the same allosteric site on the human M4 mAChRs. However, LY2119620 also binds to an allosteric site on the human M2 subtype. [(3)H]NMS ([(3)H]N-methylscopolamine) binding experiments confirm that LY2119620 does not compete for the orthosteric binding pocket at any of the five muscarinic receptor subtypes. Dissociation kinetic studies using [(3)H]NMS further support that LY2119620 binds allosterically to the M2 and M4 mAChRs and was positively cooperative with muscarinic orthosteric agonists. To probe directly the allosteric sites on M2 and M4, we radiolabeled LY2119620. Cooperativity binding of [(3)H]LY2119620 with mAChR orthosteric agonists detects significant changes in Bmax values with little change in Kd, suggesting a G protein-dependent process. Furthermore, [(3)H]LY2119620 was displaced by compounds of similar chemical structure but not by previously described mAChR allosteric compounds such as gallamine or WIN 62,577 (17-beta-hydroxy-17-alpha-ethynyl-delta-4-androstano[3,2-b]pyrimido[1,2-a]benzimi dazole). Our results therefore demonstrate the development of a radioligand, [(3)H]LY2119620 to probe specifically the human M2 and M4 muscarinic receptor allosteric binding sites.
Characterization of the novel positive allosteric modulator, LY2119620, at the muscarinic M(2) and M(4) receptors.[Pubmed:24807965]
Mol Pharmacol. 2014 Jul;86(1):106-15.
The M(4) receptor is a compelling therapeutic target, as this receptor modulates neural circuits dysregulated in schizophrenia, and there is clinical evidence that muscarinic agonists possess both antipsychotic and procognitive efficacy. Recent efforts have shifted toward allosteric ligands to maximize receptor selectivity and manipulate endogenous cholinergic and dopaminergic signaling. In this study, we present the pharmacological characterization of LY2119620 (3-amino-5-chloro-N-cyclopropyl-4-methyl-6-[2-(4-methylpiperazin-1-yl)-2-oxoethox y] thieno[2,3-b]pyridine-2-carboxamide), a M(2)/M(4) receptor-selective positive allosteric modulator (PAM), chemically evolved from hits identified through a M4 allosteric functional screen. Although unsuitable as a therapeutic due to M(2) receptor cross-reactivity and, thus, potential cardiovascular liability, LY2119620 surpassed previous congeners in potency and PAM activity and broadens research capabilities through its development into a radiotracer. Characterization of LY2119620 revealed evidence of probe dependence in both binding and functional assays. Guanosine 5'-[gamma-(35)S]-triphosphate assays displayed differential potentiation depending on the orthosteric-allosteric pairing, with the largest cooperativity observed for oxotremorine M (Oxo-M) LY2119620. Further [(3)H]Oxo-M saturation binding, including studies with guanosine-5'-[(beta,gamma)-imido]triphosphate, suggests that both the orthosteric and allosteric ligands can alter the population of receptors in the active G protein-coupled state. Additionally, this work expands the characterization of the orthosteric agonist, iperoxo, at the M(4) receptor, and demonstrates that an allosteric ligand can positively modulate the binding and functional efficacy of this high efficacy ligand. Ultimately, it was the M(2) receptor pharmacology and PAM activity with iperoxo that made LY2119620 the most suitable allosteric partner for the M(2) active-state structure recently solved (Kruse et al., 2013), a structure that provides crucial insights into the mechanisms of orthosteric activation and allosteric modulation of muscarinic receptors.


