Manassantin ACAS# 88497-87-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88497-87-4 | SDF | Download SDF |
| PubChem ID | 10190528 | Appearance | White-yellowish powder |
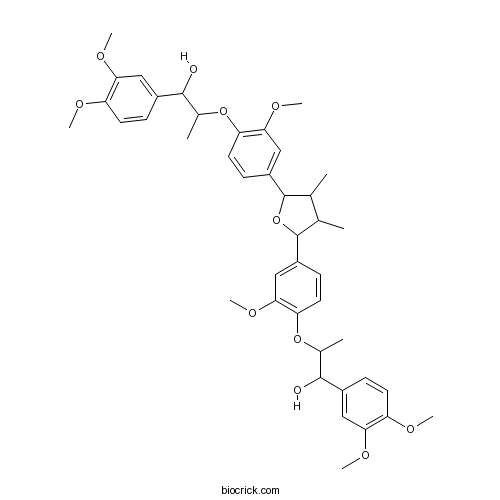
| Formula | C42H52O11 | M.Wt | 732.9 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Synonyms | Saucernetin A | ||
| Solubility | Soluble in methanol; sparingly soluble in water | ||
| Chemical Name | 1-(3,4-dimethoxyphenyl)-2-[4-[5-[4-[1-(3,4-dimethoxyphenyl)-1-hydroxypropan-2-yl]oxy-3-methoxyphenyl]-3,4-dimethyloxolan-2-yl]-2-methoxyphenoxy]propan-1-ol | ||
| SMILES | CC1C(C(OC1C2=CC(=C(C=C2)OC(C)C(C3=CC(=C(C=C3)OC)OC)O)OC)C4=CC(=C(C=C4)OC(C)C(C5=CC(=C(C=C5)OC)OC)O)OC)C | ||
| Standard InChIKey | ZGXXNVOBEIRACL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C42H52O11/c1-23-24(2)42(30-14-18-34(38(22-30)50-10)52-26(4)40(44)28-12-16-32(46-6)36(20-28)48-8)53-41(23)29-13-17-33(37(21-29)49-9)51-25(3)39(43)27-11-15-31(45-5)35(19-27)47-7/h11-26,39-44H,1-10H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Manassantin A Dilution Calculator

Manassantin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3644 mL | 6.8222 mL | 13.6444 mL | 27.2889 mL | 34.1111 mL |
| 5 mM | 0.2729 mL | 1.3644 mL | 2.7289 mL | 5.4578 mL | 6.8222 mL |
| 10 mM | 0.1364 mL | 0.6822 mL | 1.3644 mL | 2.7289 mL | 3.4111 mL |
| 50 mM | 0.0273 mL | 0.1364 mL | 0.2729 mL | 0.5458 mL | 0.6822 mL |
| 100 mM | 0.0136 mL | 0.0682 mL | 0.1364 mL | 0.2729 mL | 0.3411 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Artesunate
Catalog No.:BCN2457
CAS No.:88495-63-0
- Murraxocin
Catalog No.:BCN4431
CAS No.:88478-44-8
- (E)-FeCP-oxindole
Catalog No.:BCC6078
CAS No.:884338-18-5
- Buparvaquone
Catalog No.:BCC5437
CAS No.:88426-33-9
- 6,7-Dihydroxycoumarin-4-Acetic Acid
Catalog No.:BCC9205
CAS No.:88404-14-2
- L-(-)-α-Methyldopa hydrochloride
Catalog No.:BCC4083
CAS No.:884-39-9
- Methyl syringate
Catalog No.:BCN4430
CAS No.:884-35-5
- AZD2932
Catalog No.:BCC6388
CAS No.:883986-34-3
- 8alpha-(2-Methylacryloyloxy)-1-O-methylhirsutinolide 13-O-acetate
Catalog No.:BCN7106
CAS No.:883872-71-7
- 8-Lavandulylkaempferol
Catalog No.:BCN3961
CAS No.:883859-83-4
- Adrenorphin
Catalog No.:BCC1021
CAS No.:88377-68-8
- DPDPE
Catalog No.:BCC5758
CAS No.:88373-73-3
- Farrerol 7-O-glucoside
Catalog No.:BCN6412
CAS No.:885044-12-2
- Benzotetramisole
Catalog No.:BCC8861
CAS No.:885051-07-0
- ARRY-520 R enantiomer
Catalog No.:BCC1368
CAS No.:885060-08-2
- ARRY 520 trifluoroacetate
Catalog No.:BCC2391
CAS No.:885060-09-3
- Dichotomitin
Catalog No.:BCN8524
CAS No.:88509-91-5
- GW9508
Catalog No.:BCC1102
CAS No.:885101-89-3
- LY 2389575 hydrochloride
Catalog No.:BCC7985
CAS No.:885104-09-6
- Rosamultin
Catalog No.:BCN7391
CAS No.:88515-58-6
- W-13 hydrochloride
Catalog No.:BCC6620
CAS No.:88519-57-7
- Kongensin A
Catalog No.:BCN4432
CAS No.:885315-96-8
- MK-8745
Catalog No.:BCC3994
CAS No.:885325-71-3
- HJC 0350
Catalog No.:BCC6302
CAS No.:885434-70-8
Discovery of Manassantin A Protein Targets Using Large-Scale Protein Folding and Stability Measurements.[Pubmed:27322910]
J Proteome Res. 2016 Aug 5;15(8):2688-96.
Manassantin A is a natural product that has been shown to have anticancer activity in cell-based assays, but has a largely unknown mode-of-action. Described here is the use of two different energetics-based approaches to identify protein targets of Manassantin A. Using the stability of proteins from rates of oxidation technique with an isobaric mass tagging strategy (iTRAQ-SPROX) and the pulse proteolysis technique with a stable isotope labeling with amino acids in cell culture strategy (SILAC-PP), over 1000 proteins in a MDA-MB-231 cell lysate grown under hypoxic conditions were assayed for Manassantin A interactions (both direct and indirect). A total of 28 protein hits were identified with Manassantin A-induced thermodynamic stability changes. Two of the protein hits (filamin A and elongation factor 1alpha) were identified using both experimental approaches. The remaining 26 hit proteins were only assayed in either the iTRAQ-SPROX or the SILAC-PP experiment. The 28 potential protein targets of Manassantin A identified here provide new experimental avenues along which to explore the molecular basis of Manassantin A's mode of action. The current work also represents the first application iTRAQ-SPROX and SILAC-PP to the large-scale analysis of protein-ligand binding interactions involving a potential anticancer drug with an unknown mode-of-action.
Protective Effects of Manassantin A against Ethanol-Induced Gastric Injury in Rats.[Pubmed:26632199]
Biol Pharm Bull. 2016;39(2):221-9.
Manassantin A, a neolignan isolated from Saururus chinensis, is a major phytochemical compound that has various biological activities, including anti-inflammatory, neuroleptic, and human acyl-CoA : cholesterol acyltransferase (ACAT) inhibitory activities. In this study, we investigated the protective effects of Manassantin A against ethanol-induced acute gastric injury in rats. Gastric injury was induced by intragastric administration of 5 mL/kg body weight of absolute ethanol to each rat. The positive control group and the Manassantin A group were given oral doses of omeprazole (20 mg/kg) or Manassantin A (15 mg/kg), respectively, 1 h prior to the administration of absolute ethanol. Our examinations revealed that Manassantin A pretreatment reduced ethanol-induced hemorrhage, hyperemia, and epithelial cell loss in the gastric mucosa. Manassantin A pretreatment also attenuated the increased lipid peroxidation associated with ethanol-induced acute gastric lesions, increased the mucosal glutathione (GSH) content, and enhanced the activities of antioxidant enzymes. The levels of pro-inflammatory cytokines, tumor necrosis factor-alpha (TNF-alpha), interleukin (IL)-6, and IL-1beta were clearly decreased in the Manassantin A-pretreated group. In addition, Manassantin A pretreatment enhanced the levels of cyclooxygenase (COX)-1, COX-2, and prostaglandin E2 (PGE2) and reduced the inducible nitric oxide synthase (iNOS) overproduction and nuclear factor kappa B (NF-kappaB) phosphorylation. Collectively, these results indicate that Manassantin A protects the gastric mucosa from ethanol-induced acute gastric injury, and suggest that these protective effects might be associated with COX/PGE2 stimulation, inhibition of iNOS production and NF-kappaB activation, and improvements in the antioxidant and anti-inflammatory status.
Synthesis and Biological Evaluation of Manassantin Analogues for Hypoxia-Inducible Factor 1alpha Inhibition.[Pubmed:26394152]
J Med Chem. 2015 Oct 8;58(19):7659-71.
To cope with hypoxia, tumor cells have developed a number of adaptive mechanisms mediated by hypoxia-inducible factor 1 (HIF-1) to promote angiogenesis and cell survival. Due to significant roles of HIF-1 in the initiation, progression, metastasis, and resistance to treatment of most solid tumors, a considerable amount of effort has been made to identify HIF-1 inhibitors for treatment of cancer. Isolated from Saururus cernuus, manassantins A (1) and B (2) are potent inhibitors of HIF-1 activity. To define the structural requirements of manassantins for HIF-1 inhibition, we prepared and evaluated a series of Manassantin Analogues. Our SAR studies examined key regions of manassantin's structure in order to understand the impact of these regions on biological activity and to define modifications that can lead to improved performance and drug-like properties. Our efforts identified several Manassantin Analogues with reduced structural complexity as potential lead compounds for further development. Analogues MA04, MA07, and MA11 down-regulated hypoxia-induced expression of the HIF-1alpha protein and reduced the levels of HIF-1 target genes, including cyclin-dependent kinase 6 (Cdk6) and vascular endothelial growth factor (VEGF). These findings provide an important framework to design potent and selective HIF-1alpha inhibitors, which is necessary to aid translation of manassantin-derived natural products to the clinic as novel therapeutics for cancers.
X609, a novel manassantin A derivative, exhibits antitumor activity in MG-63 human osteosarcoma cells in vitro and in vivo.[Pubmed:25954853]
Mol Med Rep. 2015 Aug;12(2):3115-20.
Manassantin A has been well-established as an inhibitor of HIF-1. In the present study, a new manasantin A derivative, X609, with decreased stereochemical complexity, rendering it amenable to a simplified synthesis scheme, was synthesized and was found to increase HIF-1 inhibitory activity. X609 exhibited antiproliferative activity in a broad spectrum of tumor cell lines, via HIF-1-dependent mechanisms. X609 may induce apoptosis in MG-63 cells through activation of the mitochondrial pathway. Oral administration of X609 significantly inhibited the growth of human osteosarcomas implanted into nude mice. In light of the results of the present study, it may be possible to develop X609 for use as a novel antitumor agent, which targets human osteosarcoma.
LXY6090 - a novel manassantin A derivative - limits breast cancer growth through hypoxia-inducible factor-1 inhibition.[Pubmed:27445487]
Onco Targets Ther. 2016 Jun 24;9:3829-40.
Hypoxia-inducible factor-1 (HIF-1) represents a novel antitumor target owing to its involvement in vital processes considered hallmarks of cancer phenotypes. Manassantin A (MA) derived from Saururus cernuus has been reported as a selective HIF-1 inhibitor. Herein, the structure of MA was optimized to achieve new derivatives with simple chemical properties while retaining its activity. LXY6090 was designed to replace the central tetrahydrofuran moiety of MA with a cyclopentane ring and was identified as a potent HIF-1 inhibitor with an IC50 value of 4.11 nM. It not only inhibited the activity of HIF-1 in breast cancer cells but also downregulated the protein level of HIF-1alpha, which depended on von Hippel-Lindau for proteasome degradation. The related biological evaluation showed that the activity of HIF-1 target genes, VEGF and IGF-2, was decreased by LXY6090 in breast cancer cell lines. LXY6090 presented potent antitumor activity in vitro. Furthermore, LXY6090 showed in vivo anticancer efficacy by decreasing the HIF-1alpha expression in nude mice bearing MX-1 tumor xenografts. In conclusion, our data provide a basis for the future development of the novel compound LXY6090 as a potential therapeutic agent for breast cancer.


