AdrenorphinEndogenous ╬╝/╬║ opioid agonist,potent and selective CAS# 88377-68-8 |
- Adrenorphin, Free Acid
Catalog No.:BCC1011
CAS No.:88866-92-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88377-68-8 | SDF | Download SDF |
| PubChem ID | 122176 | Appearance | Powder |
| Formula | C44H69N15O9S | M.Wt | 984.18 |
| Type of Compound | N/A | Storage | Desiccate at -20┬░C |
| Synonyms | Metorphamide | ||
| Solubility | DMSO : 50 mg/mL (50.80 mM; Need ultrasonic) | ||
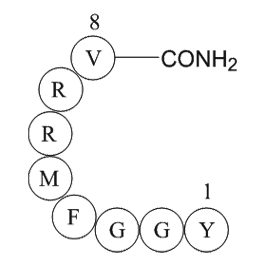
| Sequence | H2N-Tyr-Gly-Gly-Phe-Met-Arg-Arg-Val-amide | ||
| Chemical Name | (2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]acetyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-4-methylsulfanylbutanoyl]amino]-N-[(2R)-1-[[(2S)-1-amino-3-methyl-1-oxobutan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]-5-(diaminomethylideneamino)pentanamide | ||
| SMILES | CC(C)C(C(=O)N)NC(=O)C(CCCN=C(N)N)NC(=O)C(CCCN=C(N)N)NC(=O)C(CCSC)NC(=O)C(CC1=CC=CC=C1)NC(=O)CNC(=O)CNC(=O)C(CC2=CC=C(C=C2)O)N | ||
| Standard InChIKey | XJOQRTJDYAHKPY-YVWIMRNGSA-N | ||
| Standard InChI | InChI=1S/C44H69N15O9S/c1-25(2)36(37(46)63)59-41(67)31(12-8-19-52-44(49)50)57-39(65)30(11-7-18-51-43(47)48)56-40(66)32(17-20-69-3)58-42(68)33(22-26-9-5-4-6-10-26)55-35(62)24-53-34(61)23-54-38(64)29(45)21-27-13-15-28(60)16-14-27/h4-6,9-10,13-16,25,29-33,36,60H,7-8,11-12,17-24,45H2,1-3H3,(H2,46,63)(H,53,61)(H,54,64)(H,55,62)(H,56,66)(H,57,65)(H,58,68)(H,59,67)(H4,47,48,51)(H4,49,50,52)/t29-,30-,31+,32-,33-,36-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 Ōäā and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20Ōäā for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20Ōäā. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Adrenorphin Dilution Calculator

Adrenorphin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Adrenorphin (metorphamide) is a potent inhibitor of nicotine-induced adrenaline and noradrenaline release with IC50 value of 10 ╬╝M [1].
Adrenorphin (metorphamide) is an endogenous, C-terminally amidated, opioid octapeptide (Tyr-Gly-Gly-Phe-Met-Arg-Arg-Val-NH2) that is produced from proteolytic cleavage of proenkephalin A and is widely distributed throughout the mammalian brain [2].
Adrenorphin (metorphamide) is selective nicotine-induced adrenaline and noradrenaline release inhibitor and has higher than 100-fold more potent than the reported nicotine-induced adrenaline and noradrenaline release inhibitor Met5-enkephalin. When tested with bovine adrenal chromaffin cells, Adrenorphin (metorphamide) showed inhibition on 5 ╬╝M nicotine-induced ATP release by almost 50% at 5╬╝M and the inhibition was not in a dose-dependent manner [1]. In bioassays and binding assays, Metorphamide exhibited high ╬╝-binding activity, as well as ╬║-binding activity with nearly half percent to╬╝-binding activity, while showed no activity on ╬┤-binding [2].
References:
[1]. Marley, P.D., K.I. Mitchelhill, and B.G. Livett, Metorphamide, a novel endogenous adrenal opioid peptide, inhibits nicotine-induced secretion from bovine adrenal chromaffin cells. Brain Res, 1986. 363(1): p. 10-7.
[2]. Weber, E., et al., Metorphamide: isolation, structure, and biologic activity of an amidated opioid octapeptide from bovine brain. Proc Natl Acad Sci U S A, 1983. 80(23): p. 7362-6.
- DPDPE
Catalog No.:BCC5758
CAS No.:88373-73-3
- (9Z,12Z)-N-(3-Methoxybenzyl)octadeca-9,12-dienamide
Catalog No.:BCN1316
CAS No.:883715-22-8
- N-Benzyloleamide
Catalog No.:BCN1317
CAS No.:101762-87-2
- N-Benzyllinolenamide
Catalog No.:BCN6531
CAS No.:883715-18-2
- E 64d
Catalog No.:BCC1127
CAS No.:88321-09-9
- BI 78D3
Catalog No.:BCC8089
CAS No.:883065-90-5
- CCG 50014
Catalog No.:BCC4897
CAS No.:883050-24-6
- HC 067047
Catalog No.:BCC7861
CAS No.:883031-03-6
- Polygalasaponin F
Catalog No.:BCN2317
CAS No.:882664-74-6
- AMG-47A
Catalog No.:BCC6394
CAS No.:882663-88-9
- Netobimin
Catalog No.:BCC9100
CAS No.:88255-01-0
- R1530
Catalog No.:BCC1879
CAS No.:882531-87-5
- 8-Lavandulylkaempferol
Catalog No.:BCN3961
CAS No.:883859-83-4
- 8alpha-(2-Methylacryloyloxy)-1-O-methylhirsutinolide 13-O-acetate
Catalog No.:BCN7106
CAS No.:883872-71-7
- AZD2932
Catalog No.:BCC6388
CAS No.:883986-34-3
- Methyl syringate
Catalog No.:BCN4430
CAS No.:884-35-5
- L-(-)-╬▒-Methyldopa hydrochloride
Catalog No.:BCC4083
CAS No.:884-39-9
- 6,7-Dihydroxycoumarin-4-Acetic Acid
Catalog No.:BCC9205
CAS No.:88404-14-2
- Buparvaquone
Catalog No.:BCC5437
CAS No.:88426-33-9
- (E)-FeCP-oxindole
Catalog No.:BCC6078
CAS No.:884338-18-5
- Murraxocin
Catalog No.:BCN4431
CAS No.:88478-44-8
- Artesunate
Catalog No.:BCN2457
CAS No.:88495-63-0
- Manassantin A
Catalog No.:BCC8207
CAS No.:88497-87-4
- Farrerol 7-O-glucoside
Catalog No.:BCN6412
CAS No.:885044-12-2
[Approach to the study of natural peptides from structure to functions. III. Structural organization of an adrenorphin molecule and its synthetic analogs].[Pubmed:8363653]
Bioorg Khim. 1993 Jun;19(6):623-8.
Spatial structure of the peptide hormone Adrenorphin was investigated by the theoretical conformational method. A solution of the "reverse conformational problem" for Adrenorphin made it possible to predict a series of the modified synthetic analogues, which may assume one of the low-energy conformations of the native hormone.
Distribution of immunoreactive metorphamide (adrenorphin) in discrete regions of the rat brain: comparison with Met-enkephalin-Arg6-Gly7-Leu8.[Pubmed:4084793]
Brain Res. 1985 Dec 30;361(1-2):193-9.
The distribution of immunoreactive (ir)-metorphamide (Adrenorphin) in 101 microdissected rat brain and spinal cord regions was determined using a highly specific radioimmunoassay. The highest concentration of metorphamide in brain was found in globus pallidus (280.1 fmol/mg protein). High concentrations of ir-metorphamide (greater than 120 fmol/mg protein) were found in 9 nuclei, including central amygdaloid nucleus, lateral preoptic area, anterior hypothalamic nucleus, hypothalamic paraventricular nucleus, interpeduncular nucleus, periaqueductal grey matter and nucleus of the solitary tract. Moderate concentrations of the peptide (between 60 and 120 fmol/mg protein) were found in 47 brain nuclei such as nucleus accumbens, bed nucleus of stria terminalis, several septal and amygdaloid nuclei, most of the hypothalamic nuclei, ventral tegmental area, red nucleus, raphe nuclei, lateral reticular nucleus, area postrema and others. Low concentrations or ir-metorphamide (less than 60 fmol/mg protein) were measured in 41 nuclei, e.g., cortical structures, hippocampus, caudate nucleus, thalamic nuclei, supraoptic nucleus, substantia nigra, vestibular nuclei, cerebellum (nuclei and cortex). The olfactory bulb has the lowest metorphamide concentration (5.8 fmol/mg protein). Spinal cord segments exhibit very low peptide concentrations.
Studies on adrenorphin in pheochromocytoma.[Pubmed:3818899]
J Clin Endocrinol Metab. 1987 Apr;64(4):692-7.
We studied the secretion and tissue contents of Adrenorphin in human pheochromocytomas. In 17 human pheochromocytomas from 11 patients, we found a remarkably wide distribution in immunoreactive Adrenorphin levels (3-7771 pg/mg tissue). Adrenomedullary pheochromocytomas contained a significantly larger amount of immunoreactive Adrenorphin (2295 +/- 1092 pg/mg, mean +/- SE) than did extramedullary ones (17.8 +/- 8.4 pg/mg). Gel chromatographic studies revealed that immunoreactive Adrenorphin consisted largely of material emerging at the position of synthetic Adrenorphin in both pheochromocytoma and normal adrenal medulla tissue. Nicotine (10(-5) M) significantly stimulated the secretion of immunoreactive Adrenorphin as well as catecholamines from cultured human pheochromocytoma cells. Adrenorphin was a more potent inhibitor of catecholamine secretion evoked by 10(-5) M nicotine than was met-enkephalin in cultured human pheochromocytoma cells. The 50% inhibitory concentrations (IC50) were 1.1 X 10(-6) and 6.5 X 10(-5) M for Adrenorphin and met-enkephalin, respectively. The effect of Adrenorphin was much the same as that of dynorphin-(1-13) (IC50, 1.0 X 10(-6) M) and BAM-12P (IC50, 4.5 X 10(-6) M). These results indicate the presence and secretion of Adrenorphin in human pheochromocytomas. Adrenorphin may play an important role in regulating catecholamine secretion in human pheochromocytoma.


