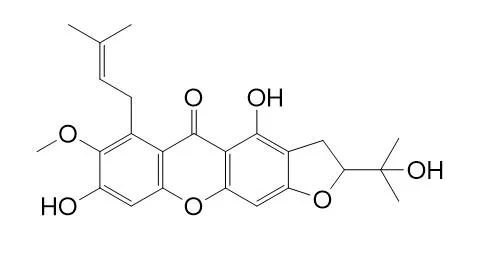
MangostaninCAS# 463342-39-4 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 463342-39-4 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C24H26O7 | M.Wt | 426.5 |
| Type of Compound | Xanthones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Mangostanin Dilution Calculator

Mangostanin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3447 mL | 11.7233 mL | 23.4467 mL | 46.8933 mL | 58.6166 mL |
| 5 mM | 0.4689 mL | 2.3447 mL | 4.6893 mL | 9.3787 mL | 11.7233 mL |
| 10 mM | 0.2345 mL | 1.1723 mL | 2.3447 mL | 4.6893 mL | 5.8617 mL |
| 50 mM | 0.0469 mL | 0.2345 mL | 0.4689 mL | 0.9379 mL | 1.1723 mL |
| 100 mM | 0.0234 mL | 0.1172 mL | 0.2345 mL | 0.4689 mL | 0.5862 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Hydroxy-2-methoxybenzoic acid
Catalog No.:BCX2145
CAS No.:90111-34-5
- 15-O-Methylgraciliflorin F
Catalog No.:BCX2144
CAS No.:1411994-51-8
- Hydrastinine
Catalog No.:BCX2143
CAS No.:5936-29-8
- Notoginsenoside E
Catalog No.:BCX2142
CAS No.:193976-50-0
- 5-Allyl-1-methoxy-2,3-dihydroxybenzene
Catalog No.:BCX2141
CAS No.:4055-72-5
- Auranamide
Catalog No.:BCX2140
CAS No.:740813-53-0
- Terrestriamide
Catalog No.:BCX2139
CAS No.:157536-49-7
- Quercetin-5-O-glucoside-3-O-rutinoside
Catalog No.:BCX2138
CAS No.:1478622-04-6
- Praeroside I
Catalog No.:BCX2137
CAS No.:121064-73-1
- Cryptostigmin II
Catalog No.:BCX2136
CAS No.:50906-57-5
- Davidigenin
Catalog No.:BCX2135
CAS No.:23130-26-9
- Pinocembrin 7-O-neohesperidoside
Catalog No.:BCX2134
CAS No.:13241-31-1
- Monoisovalerate
Catalog No.:BCX2147
CAS No.:95486-32-1
- Ajugamarin A1
Catalog No.:BCX2148
CAS No.:78798-40-0
- Obtusichromoneside B
Catalog No.:BCX2149
CAS No.:2414481-39-1
- 3,4-Dihydroxy-5-methoxybenzoic acidmethylester
Catalog No.:BCX2150
CAS No.:3934-86-9
- Leonoside F
Catalog No.:BCX2151
CAS No.:1360075-79-1
- (6-O-p-coumaroyl)-beta-glucopyranosyl-2-O-(4-hydroxybenzoyl)-4-O-beta-glucopyranosyl-6-hydroxyphenylacetate
Catalog No.:BCX2152
CAS No.:1987871-73-7
- rel-(7R,8'R,8S)-forsythialan C
Catalog No.:BCX2153
CAS No.:2089054-57-7
- Samioside
Catalog No.:BCX2154
CAS No.:360768-68-9
- Sanleng acid
Catalog No.:BCX2155
CAS No.:163860-24-0
- Clerodendroside (Scutellarein 4'-methyl ether 7-glucuronide)
Catalog No.:BCX2156
CAS No.:64924-06-7
- Tetrandrine 2' alpha-oxide (Tetrandrine-2'-N-alpha-oxide)
Catalog No.:BCX2157
CAS No.:90365-80-3
- Acetyl-11 alpha-methoxy-beta-boswellic acid
Catalog No.:BCX2158
CAS No.:918548-43-3
Mangostanin hyaluronic acid hydrogel as an effective biocompatible alternative to chlorhexidine.[Pubmed:39216568]
Int J Biol Macromol. 2024 Nov;279(Pt 1):135187.
Periodontal disease (PD) prevention and treatment products typically demonstrate excellent antibacterial activity, but recent studies have raised concerns about their toxicity on oral tissues. Therefore, finding a biocompatible alternative that retains antimicrobial properties is imperative. In this study, a chemically modified hyaluronic acid (HA) hydrogel containing Mangostanin (MGTN) was developed. Native HA was chemically modified, incorporating amino and aldehyde groups in different batches of HA, allowing spontaneous crosslinking and gelation when combined at room temperature. MGTN at different concentrations was incorporated before gelation. The structure, swelling characteristics MGTN release, rheological parameters, and in vitro degradation performance of the loaded hydrogel were first evaluated in the study. Then, antimicrobial properties were tested on Porphyromonas gingivalis and its biocompatibility in 3D-engineered human gingiva. HA hydrogel was very stable and showed a sustained release for MGTN for at least 7 days. MGTN-loaded HA hydrogel showed equivalent antimicrobial activity compared to a commercial gel of HA containing 0.2 % chlorhexidine (CHX). In contrast, while MGTN HA hydrogel was biocompatible, CHX gel showed high cytotoxicity, causing cell death and tissue damage. Modified HA hydrogel allows controlled release of MGTN, resulting in a highly biocompatible hydrogel with antibacterial properties. This hydrogel is a suitable alternative therapy to prevent and treat PD.
In Vitro Evaluation of Mangostanin as an Antimicrobial and Biocompatible Topical Antiseptic for Skin and Oral Tissues.[Pubmed:38751630]
ACS Pharmacol Transl Sci. 2024 Apr 19;7(5):1507-1517.
Skin and oral tissue infections pose significant health challenges worldwide, necessitating the exploration of new antiseptic agents that are both effective and biocompatible. This study evaluated the antibacterial efficacy and biocompatibility of Mangostanin (MGTN), a xanthone derived from Garcinia mangostana L., against commercial antiseptics across various bacterial strains (Porphyromonas gingivalis, Streptococcus mutans, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Cutibacterium acnes) and in vitro models of skin and oral tissues. MGTN demonstrated significant antimicrobial activity against all tested pathogens concurrently exhibiting negligible cytotoxic effects on human gingival fibroblasts as well as on three-dimensional (3D) models of human epidermis and oral epithelium. Furthermore, using pooled human saliva, MGTN effectively inhibited plaque biofilm formation, suggesting its potential as a natural, biocompatible antiseptic for skin and oral health applications. These findings position MGTN as a promising candidate for further development into antiseptic formulations, offering a natural alternative to current synthetic options.
Discovery of novel PDE4 inhibitors targeting the M-pocket from natural mangostanin with improved safety for the treatment of Inflammatory Bowel Diseases.[Pubmed:35985255]
Eur J Med Chem. 2022 Nov 15;242:114631.
Inflammatory Bowel Diseases (IBDs) are chronic disorders with iterative intestinal mucosal inflammation which remain unmet medical needs. PDE4 inhibitors were reported to be novel anti-IBD agents, but their clinical use was hampered by side effects such as emesis and nausea. Herein, structure-based discovery of natural Mangostanin (1) targeting the M-pocket resulted in the novel and potent PDE4 inhibitor 22d (IC(50) = 3.5 nM) and favorable physico-chemical properties. X-Ray study revealed that 22d interacted tightly with the M-pocket and maintained the key interactions between PDE4 and roflumilast. Worthy to note that compounds 22d and our previously reported 4e and 18a, originating from Mangostanin, all caused no emesis on beagle dogs at the oral dose of 10 mg/kg, confirming the safety superiority of scaffold in Mangostanin derivatives over that in positive roflumilast. Finally, administration of 22d (5.0 mg/kg, twice-daily) exhibited comparable anti-IBD effects to the positive control dipyridamole (25.0 mg/kg, twice-daily) in the dextran sulfate sodium (DSS)-induced IBD mice model, indicating its potential as a novel anti-IBD agent.
Garcinia mangostana L. fruits and derived food supplements: Identification and quantitative determination of bioactive xanthones by NMR analysis.[Pubmed:35659659]
J Pharm Biomed Anal. 2022 Sep 5;218:114835.
Mangosteen (Garcinia mangostana L.), known as "the queen of fruits", is one of the most praised tropical fruit due to its delicious taste. In the last years, the use of mangosteen in functional products has been increasing, mainly in food beverages and nutraceutical formulations due to its biological activities related to the content of xanthones. The quantitative Nuclear Magnetic Resonance (qNMR) analysis, a rapid and accurate method used for simultaneous quantification of plant metabolites, was here employed to determine the amount of bioactive xanthones in the extracts of G. mangostana arils and shells obtained by using solvent of increasing polarity along with ''eco-friendly'' solvents like ethanol and ethanol-water. Furthermore, the content of xanthones was compared with that occurring in four selected commercial food supplements, among which tablets and capsules, and two fruit juices, based on mangosteen. Quantitative results highlighted a significant variability: the extracts of the shells displayed a higher amount of bioactive xanthones than those of the arils, in particular, of gamma-mangostin and alpha-mangostin, while beta-mangostin, demethylcalabaxanthone, Mangostanin, 8-deoxygartanin occurred in higher amounts in arils. A certain variability in the amount of biologically active xanthones (i.e. alpha-mangostin and gamma-mangostin) could be observed in commercial food supplements.
Mangostanin, a Xanthone Derived from Garcinia mangostana Fruit, Exerts Protective and Reparative Effects on Oxidative Damage in Human Keratinocytes.[Pubmed:35056141]
Pharmaceuticals (Basel). 2022 Jan 11;15(1):84.
The fruit of Garcinia mangostana (mangosteen) is known in ancient traditional Asian medicine for its antioxidant, anti-inflammatory, immunomodulatory and anticancer activities. These effects are mainly due to the action of polyphenols known as xanthones, which are contained in the pericarp of the fruit. In recent years, there has been a growing interest from pharmaceutical companies in formulating new topicals based on mangosteen full extracts to prevent skin aging. However, the molecules responsible for these effects and the mechanisms involved have not been investigated so far. Here, the arils and shells of Garcinia mangostana were extracted with chloroform and methanol, and the extracts were further purified to yield 12 xanthone derivatives. Their effects were evaluated using in vitro cultures of human epidermal keratinocytes. After confirming the absence of cytotoxicity, we evaluated the antioxidant potential of these compounds, identifying Mangostanin as capable of both protecting and restoring oxidative damage induced by H(2)O(2). We showed how Mangostanin, by reducing the generation of intracellular reactive oxygen species (ROS), prevents the activation of AKT (protein kinase B), ERK (extracellular signal-regulated kinase), p53, and other cellular pathways underlying cell damage and apoptosis activation. In conclusion, our study is the first to demonstrate that Mangostanin is effective in protecting the skin from the action of free radicals, thus preventing skin aging, confirming a potential toward its development in the nutraceutical and cosmeceutical fields.
Mangostanin Derivatives as Novel and Orally Active Phosphodiesterase 4 Inhibitors for the Treatment of Idiopathic Pulmonary Fibrosis with Improved Safety.[Pubmed:34520193]
J Med Chem. 2021 Sep 23;64(18):13736-13751.
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease, and its incidence rate is rapidly rising. However, effective therapies for the treatment of IPF are still lacking. Phosphodiesterase 4 (PDE4) inhibitors were reported to be potential anti-fibrotic agents, but their clinical use was hampered by side effects like emesis and nausea. Herein, structure-based hit-to-lead optimizations of natural Mangostanin resulted in the novel and orally active PDE4 inhibitor 18a with potent inhibitory affinity (IC(50) = 4.2 nM), favorable physico-chemical properties, and a different binding pattern from roflumilast. Emetic activity tests on dogs demonstrated that 18a cannot cause emesis even at an oral dose of 10 mg/kg, whereas rolipram had severe emetic effects at an oral dose of 1 mg/kg. Finally, the oral administration of 18a (10 mg/kg) exhibited comparable anti-pulmonary fibrosis effects with pirfenidone (150 mg/kg) in a bleomycin-induced IPF rat model, indicating its potential as a novel anti-IPF agent with improved safety.
Two new chemical constituents from the stem bark of Garcinia mangostana.[Pubmed:24901833]
Molecules. 2014 Jun 4;19(6):7308-16.
A detailed chemical study on the ethyl acetate and methanol extracts of the stem bark of Garcinia mangostana resulted in the successful isolation of one new prenylated xanthone, mangaxanthone B (1), one new benzophenone, mangaphenone (2), and two known xanthones, Mangostanin (3) and mangostenol (4). The structures of these compounds were elucidated through analysis of their spectroscopic data obtained using 1D and 2D NMR and MS techniques.
Microbial metabolism of alpha-mangostin isolated from Garcinia mangostana L.[Pubmed:21377704]
Phytochemistry. 2011 Jun;72(8):730-4.
alpha-Mangostin (1), a prenylated xanthone isolated from the fruit hull of Garcinia mangostana L., was individually metabolized by two fungi, Colletotrichum gloeosporioides (EYL131) and Neosartorya spathulata (EYR042), repectively. Incubation of 1 with C. gloeosporioides (EYL131) gave four metabolites which were identified as mangostin 3-sulfate (2), Mangostanin 6-sulfate (3), 17,18-dihydroxyMangostanin 6-sulfate (4)and isoMangostanin 3-sulfate (5). Compound 2 was also formed by incubation with N. spathulata (EYR042). The structures of the isolated compounds were elucidated by spectroscopic data analysis. Of the isolated metabolites, 2 exhibited significant anti-mycobacterial activity against Mycobacterium tuberculosis.
Cytotoxic xanthone constituents of the stem bark of Garcinia mangostana (mangosteen).[Pubmed:19839614]
J Nat Prod. 2009 Nov;72(11):2028-31.
Bioassay-guided fractionation of a chloroform-soluble extract of Garcinia mangostana stem bark, using the HT-29 human colon cancer cell line and an enzyme-based ELISA NF-kappaB assay, led to the isolation of a new xanthone, 11-hydroxy-3-O-methyl-1-isomangostin (1). The structure of 1 was elucidated by spectroscopic data analysis. In addition, 10 other known compounds, 11-hydroxy-1-isomangostin (2), 11alpha-Mangostanin (3), 3-isomangostin (4), alpha-mangostin (5), beta-mangostin (6), garcinone D (7), 9-hydroxycalabaxanthone (8), 8-deoxygartanin (9), gartanin (10), and cratoxyxanthone (11), were isolated. Compounds 4-8 exhibited cytotoxicity against the HT-29 cell line with ED50 values of 4.9, 1.7, 1.7, 2.3, and 9.1 microM, respectively. In an ELISA NF-kappaB assay, compounds 5-7, 9, and 10 inhibited p65 activation with IC50 values of 15.9, 12.1, 3.2, 11.3, and 19.0 microM, respectively, and 6 showed p50 inhibitory activity with an IC50 value of 7.5 microM. Alpha-mangostin (5) was further tested in an in vivo hollow fiber assay, using HT-29, LNCaP, and MCF-7 cells, but it was found to be inactive at the highest dose tested (20 mg/kg).
Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen).[Pubmed:17991497]
Phytochemistry. 2008 Feb;69(3):754-8.
Bioactivity-guided fractionation of a dichloromethane-soluble extract of Garcinia mangostana fruits has led to the isolation and identification of five compounds, including two xanthones, 1,2-dihydro-1,8,10-trihydroxy-2-(2-hydroxypropan-2-yl)-9-(3-methylbut-2-enyl)furo[3,2-a]xanthen-11-one (1) and 6-deoxy-7-demethylMangostanin (2), along with three known compounds, 1,3,7-trihydroxy-2,8-di-(3-methylbut-2-enyl)xanthone (3), Mangostanin (4), and alpha-mangostin (5). The structures of compounds 1 and 2 were determined from analysis of their spectroscopic data. All isolated compounds in the present study together with eleven other compounds previously isolated from the pericarp of mangosteen, were tested in an in vitro quinone reductase-induction assay using murine hepatoma cells (Hepa 1c1c7) and an in vitro hydroxyl radical antioxidant assay. Of these, compounds 1-4 induced quinone reductase (concentration to double enzyme induction, 0.68-2.2microg/mL) in Hepa 1c1c7 cells and gamma-mangostin (6) exhibited hydroxyl radical-scavenging activity (IC50, 0.20microg/mL).


