DavidigeninCAS# 23130-26-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

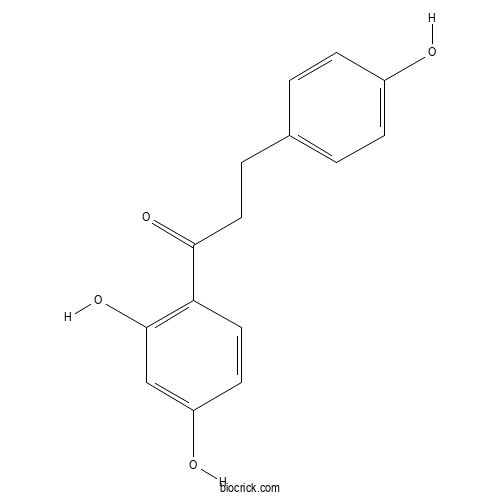
| Cas No. | 23130-26-9 | SDF | Download SDF |
| PubChem ID | 442342 | Appearance | Powder |
| Formula | C15H14O4 | M.Wt | 258.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(2,4-dihydroxyphenyl)-3-(4-hydroxyphenyl)propan-1-one | ||
| SMILES | C1=CC(=CC=C1CCC(=O)C2=C(C=C(C=C2)O)O)O | ||
| Standard InChIKey | UDGKKUWYNITJRX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H14O4/c16-11-4-1-10(2-5-11)3-8-14(18)13-7-6-12(17)9-15(13)19/h1-2,4-7,9,16-17,19H,3,8H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Davidigenin Dilution Calculator

Davidigenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8715 mL | 19.3573 mL | 38.7147 mL | 77.4293 mL | 96.7867 mL |
| 5 mM | 0.7743 mL | 3.8715 mL | 7.7429 mL | 15.4859 mL | 19.3573 mL |
| 10 mM | 0.3871 mL | 1.9357 mL | 3.8715 mL | 7.7429 mL | 9.6787 mL |
| 50 mM | 0.0774 mL | 0.3871 mL | 0.7743 mL | 1.5486 mL | 1.9357 mL |
| 100 mM | 0.0387 mL | 0.1936 mL | 0.3871 mL | 0.7743 mL | 0.9679 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pinocembrin 7-O-neohesperidoside
Catalog No.:BCX2134
CAS No.:13241-31-1
- Gerardianin A
Catalog No.:BCX2133
CAS No.:137171-31-4
- Euphopiloside A
Catalog No.:BCX2132
CAS No.:1610615-04-7
- Graciliflorin F
Catalog No.:BCX2131
CAS No.:1413941-67-9
- Eugenol 4-O-beta-D-(6'-O-galloyl) glucopyranoside
Catalog No.:BCX2130
CAS No.:152041-15-1
- Plumbagic acid
Catalog No.:BCX2129
CAS No.:75640-06-1
- Etuycomanol
Catalog No.:BCX2128
CAS No.:84633-28-3
- Ellagic acid glucoside
Catalog No.:BCX2127
CAS No.:163774-64-9
- Isorhamnetin 3-sambubioside
Catalog No.:BCX2126
CAS No.:142059-76-5
- 3'-Methoxynyasin
Catalog No.:BCX2125
CAS No.:1685246-20-1
- 8-C-beta-D-(6-O-galloyl)glucosylnoreugenin
Catalog No.:BCX2124
CAS No.:152041-17-3
- Callicapoic acid M1
Catalog No.:BCX2123
CAS No.:2756970-65-5
- Cryptostigmin II
Catalog No.:BCX2136
CAS No.:50906-57-5
- Praeroside I
Catalog No.:BCX2137
CAS No.:121064-73-1
- Quercetin-5-O-glucoside-3-O-rutinoside
Catalog No.:BCX2138
CAS No.:1478622-04-6
- Terrestriamide
Catalog No.:BCX2139
CAS No.:157536-49-7
- Auranamide
Catalog No.:BCX2140
CAS No.:740813-53-0
- 5-Allyl-1-methoxy-2,3-dihydroxybenzene
Catalog No.:BCX2141
CAS No.:4055-72-5
- Notoginsenoside E
Catalog No.:BCX2142
CAS No.:193976-50-0
- Hydrastinine
Catalog No.:BCX2143
CAS No.:5936-29-8
- 15-O-Methylgraciliflorin F
Catalog No.:BCX2144
CAS No.:1411994-51-8
- 4-Hydroxy-2-methoxybenzoic acid
Catalog No.:BCX2145
CAS No.:90111-34-5
- Mangostanin
Catalog No.:BCX2146
CAS No.:463342-39-4
- Monoisovalerate
Catalog No.:BCX2147
CAS No.:95486-32-1
Rapid biotransformation of STW 5 constituents by human gut microbiome from IBS- and non-IBS donors.[Pubmed:38738925]
Microbiol Spectr. 2024 Jun 4;12(6):e0403123.
STW 5, a blend of nine medicinal plant extracts, exhibits promising efficacy in treating functional gastrointestinal disorders, notably irritable bowel syndrome (IBS). Nonetheless, its effects on the gastrointestinal microbiome and the role of microbiota on the conversion of its constituents are still largely unexplored. This study employed an experimental ex vivo model to investigate STW 5's differential effects on fecal microbial communities and metabolite production in samples from individuals with and without IBS. Using 560 fecal microcosms (IBS patients, n = 6; healthy controls, n = 10), we evaluated the influence of pre-digested STW 5 and controls on microbial and metabolite composition at time points 0, 0.5, 4, and 24 h. Our findings demonstrate the potential of this ex vivo platform to analyze herbal medicine turnover within 4 h with minimal microbiome shifts due to abiotic factors. While only minor taxonomic disparities were noted between IBS- and non-IBS samples and upon treatment with STW 5, rapid metabolic turnover of STW 5 components into specific degradation products, such as 18beta-glycyrrhetinic acid, Davidigenin, herniarin, 3-(3-hydroxyphenyl)propanoic acid, and 3-(2-hydroxy-4-methoxyphenyl)propanoic acid occurred. For Davidigenin, 3-(3-hydroxyphenyl)propanoic acid and 18beta-glycyrrhetinic acid, anti-inflammatory, cytoprotective, or spasmolytic activities have been previously described. Notably, the microbiome-driven metabolic transformation did not induce a global microbiome shift, and the detected metabolites were minimally linked to specific taxa. Observed biotransformations were independent of IBS diagnosis, suggesting potential benefits for IBS patients from biotransformation products of STW 5. IMPORTANCE: STW 5 is an herbal medicinal product with proven clinical efficacy in the treatment of functional gastrointestinal disorders, like functional dyspepsia and irritable bowel syndrome (IBS). The effects of STW 5 on fecal microbial communities and metabolite production effects have been studied in an experimental model with fecal samples from individuals with and without IBS. While only minor taxonomic disparities were noted between IBS- and non-IBS samples and upon treatment with STW 5, rapid metabolic turnover of STW 5 components into specific degradation products with reported anti-inflammatory, cytoprotective, or spasmolytic activities was observed, which may be relevant for the pharmacological activity of STW 5.
Influence of Ultra-High-Pressure Pretreatment Method on Chemical Constituents, Antioxidant and Cytoprotective Activities of Free, Esterified, and Bound Phenolics from Anneslea Fragrans Wall. Leaves.[Pubmed:37266882]
Plant Foods Hum Nutr. 2023 Jun;78(2):407-418.
Anneslea fragrans Wall., an edible and medicinal plant, is traditionally used to treat liver and gastrointestinal diseases. This paper aimed to investigate the influence of ultra-high pressure (UHP) pretreatment on the phenolics profiling, antioxidant, and cytoprotective activities of free (FP), esterified (EP), and bound (BP) phenolics from A. fragrans leaves. A total of 32 compounds were characterized and quantified. The Davidigenin (44.46 and 113.37 mg/g extract) was the highest in A. fragrans leaves. The vitexin (9), afzelin (10), coreopsin (15), and Davidigenin (28) were analyzed with MS(2) fragment pathways. Results showed that UHP treated A. fragrans leaves had higher total phenolic (TPC) and total flavonoid (TFC) contents of FP, EP, and BP fractions than those in the raw leaves. Moreover, UHP pretreated A. fragrans leaves had higher scavenging activities on DPPH(+)* and ABTS(+)*, and inhibitory effects on the intracellular ROS generation in H(2)O(2)-induced HepG2 cells. UFP showed the highest inhibition of ROS production among the samples. Therefore, UHP pretreatment method might be used as an effective strategy for elevating the availabilities of A. fragrans leaves to develop functional foods.
Biotransformation of Liquiritigenin into Characteristic Metabolites by the Gut Microbiota.[Pubmed:35630532]
Molecules. 2022 May 10;27(10):3057.
The bioavailability of flavonoids is generally low after oral administration. The metabolic transformation of flavonoids by the gut microbiota may be one of the main reasons for this, although these metabolites have potential pharmacological activities. Liquiritigenin is an important dihydroflavonoid compound found in Glycyrrhiza uralensis that has a wide range of pharmacological properties, such as antitumor, antiulcer, anti-inflammatory, and anti-AIDS effects, but its mechanism of action remains unclear. This study explored the metabolites of liquiritigenin by examining gut microbiota metabolism and hepatic metabolism in vitro. Using LC-MS/MS and LC/MS(n)-IT-TOF techniques, three possible metabolites of liquiritigenin metabolized by the gut microbiota were identified: phloretic acid (M3), resorcinol (M4), and M5. M5 is speculated to be Davidigenin, which has antitumor activity. By comparing these two metabolic pathways of liquiritigenin (the gut microbiota and liver microsomes), this study revealed that there are three main metabolites of liquiritigenin generated by intestinal bacteria, which provides a theoretical basis for the study of pharmacologically active substances in vivo.
Characterization of PMI-5011 on the Regulation of Deubiquitinating Enzyme Activity in Multiple Myeloma Cell Extracts.[Pubmed:33716550]
Biochem Eng J. 2021 Feb;166:107834.
Deubiquitinating enzyme (DUB)-targeted therapeutics have shown promise in recent years as alternative cancer therapeutics, especially when coupled with proteasome-based inhibitors. While a majority of DUB-based therapeutics function by inhibiting DUB enzymes, studies show that positive regulation of these enzymes can stabilize levels of protein degradation. Unfortunately, there are currently no clinically available therapeutics for this purpose. The goal of this work was to understand the effect of a botanical extract from Artemisia dracunculus L called PMI-5011 on DUB activity in cancer cells. Through a series of kinetic analyses and mathematical modeling, it was found that PMI-5011 positively regulated DUB activity in two model multiple myeloma cells line (OPM2 and MM.1S). This suggests that PMI-5011 interacts with the active domains of DUBs to enhance their activity directly or indirectly, without apparently affecting cellular viability. Similar kinetic profiles of DUB activity were observed with three bioactive compounds in PMI-5011 (DMC-1, DMC-2, Davidigenin). Interestingly, a differential cell line-independent trend was observed at higher concentrations which suggested variances in inherent gene expressions of UCHL1, UCHL5, USP7, USP15, USP14, and Rpn11 in OPM2 and MM.1S cell lines. These findings highlight the therapeutic potential of PMI-5011 and its selected bioactive compounds in cancer.
From genomes to molecular dynamics - A bottom up approach in extrication of SARS CoV-2 main protease inhibitors.[Pubmed:33532671]
Comput Toxicol. 2021 May;18:100156.
The recent pandemic Coronavirus disease-19 outbreak had traumatized global countries since its origin in late December 2019. Though the virus originated in China, it has spread rapidly across the world due its firmly established community transmission. To successfully tackle the spread and further infection, there needs a clear multidimensional understanding of the molecular mechanisms. Henceforth, 942 viral genome sequences were analysed to predict the core genomes crucial in virus life cycle. Additionally, 35 small interfering RNA transcripts were predicted that can target specifically the viral core proteins and reduce pathogenesis. The crystal structure of Covid-19 main protease-6LU7 was chosen as an attractive target due to the factors that there were fewer mutations and whose structure had significant identity to the annotated protein sequence of the core genome. Drug repurposing of both recruiting and non recruiting drugs was carried out through molecular docking procedures to recognize bitolterol as a good inhibitor of Covid-19 protease. The study was extended further to screen antiviral phytocompounds through quantitative structure activity relationship and molecular docking to identify Davidigenin, from licorice as the best novel lead with good interactions and binding energy. The docking of the best compounds in all three categories was validated with molecular dynamics simulations which implied stable binding of the drug and lead molecule. Though the studies need clinical evaluations, the results are suggestive of curbing the pandemic.
The influence of compatibility of Si-Ni decoction with metabolism in intestinal bacteria on transports of toxic diterpenoid alkaloids from processed aconite root across Caco-2 monolayers.[Pubmed:30223050]
J Ethnopharmacol. 2019 Jan 10;228:164-178.
ETHNOPHARMACOLOGICAL RELEVANCE: In traditional Chinese medicine, processed aconite root (lateral root of Aconitum carmichaelii Debx.) is used as the principle herb of the Si-Ni decoction (SND) formula due to its cardiotonic effect, while its cardiotoxicity and neurotoxicity caused by diester and monoester diterpenoid alkaloids are reduced by compatibility of dried ginger and honey-processed liquorice in SND. AIM OF THE STUDY: To investigate the detoxification of processed aconite root by compatibility of SND from the perspective of intestinal absorption with metabolism in intestinal bacteria. MATERIALS AND METHODS: Decoctions of processed aconite root (AD), processed aconite root and honey-processed liquorice (ALD), and SND with the same amount of each herb were prepared, then were incubated in human intestinal bacteria juice (IBJ) in vitro for different durations. Bidirectional transmembrane transports of these decoctions and their IBJ-incubated decoctions were conducted on Caco-2 monolayers. Correlation between efflux ratios changes of benzoylmesaconine, benzoylaconine, benzoylhypaconine (monoester-diterpenoid alkaloids, MDAs) and hypaconitine (diester-diterpenoid alkaloids) from processed aconite root, and metabolic trends of compounds from honey-processed liquorice and dried ginger were also performed. RESULTS: The absorption of MDAs from processed aconite root was increased by combination with honey-processed liquorice in ALD, but they were decreased significantly by the addition of dried ginger in SND. Take benzoylhypaconine for example, the P(app, AP to BL) soared from (3.13 +/- 0.18) x 10(-7) cm/s in AD to (23.32 +/- 3.51) x 10(-7) cm/s in ALD, while it dropped to (1.12 +/- 0.17) x 10(-7) cm/s in SND. When herb combined decoctions metabolised by intestinal bacteria for 12 h, the efflux ratio of benzoylhypaconine were both increased from 0.56 to 1.21 in ALD and from 1.10 to 2.61 in SND, which was correlative with the generation of Davidigenin and glycyrrhetic acid (the metabolites of chalcones and pentacyclic triterpenoids from liquorice) in ALD and with the metabolism of [6]-gingerol (the major compound from dried ginger) in SND, respectively. CONCLUSIONS: Compatibility of SND altered the intestinal absorption of toxic MDAs and hypaconitine from processed aconite root. In SND, dried ginger rather than honey-processed liquorice played the role of detoxification of these toxic compounds in the intestinal absorption. The intestinal detoxification of SND was significantly and strongly correlative with metabolism of dried ginger and honey-processed liquorice by intestinal bacteria, simultaneously.
Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential Against alpha-Amylase and alpha-Glucosidase.[Pubmed:30050443]
Front Pharmacol. 2018 Jul 12;9:756.
Artemisia genus of Asteraceae family is a source of medicinal plants known worldwide and used as ethnopharmacological remedies for the treatment of diabetes in Northern Asia (Siberia). The aim of this study was to determine the phenolic profile of 12 Siberian Artemisia species (A. anethifolia, A. commutata, A. desertorum, A. integrifolia, A. latifolia, A. leucophylla, A. macrocephala, A. messerschmidtiana, A. palustris, A. sericea, A. tanacetifolia, A. umbrosa) and to test the efficacy of plant extracts and pure compounds for antidiabetic potential. Finally, by HPLC-DAD-ESI-TQ-MS/MS technique, 112 individual phenolic compounds were detected in Artemisia extracts in a wide range of concentrations. Some species accumulated rare plant phenolics, such as coumarin-hemiterpene ethers (lacarol derivatives) from A. latifolia and A. tanacetifolia; melilotoside from A. tanacetifolia; dihydrochalcones (Davidigenin analogs) from A. palustris; chrysoeriol glucosides from A. anethifolia, A. sericea, and A. umbrosa; eriodictyol glycosides from A. messerschmidtiana; and some uncommon flavones and flavonols. The predominant phenolic group from Artemisia species herb was caffeoylquinic acid (CQAs), and in all species, the major CQAs were 5-O-CQA (20.28-127.99 mug/g) and 3,5-di-O-CQA (7.35-243.61 mug/g). In a series of in vitro bioassays, all studied Artemisia extracts showed inhibitory activity against principal enzymes of carbohydrate metabolism, such as alpha-amylase (IC(50) = 150.24-384.14 mug/mL) and alpha-glucosidase (IC(50) = 214.42-754.12 mug/mL). Although many phenolic compounds can be inhibitors, experimental evidence suggests that the CQAs were key to the biological response of Artemisia extracts. Mono-, di- and tri-substituted CQAs were assayed and showed inhibition of alpha-amylase and alpha-glucosidase, with IC(50) values of 40.57-172.47 muM and 61.08-1240.35 muM, respectively, and they were more effective than acarbose, a well-known enzyme inhibitor. The results obtained in this study reveal that Siberian Artemisia species and CQAs possess a pronounced inhibitory activity against alpha-amylase and alpha-glucosidase and could become a complement to synthetic antidiabetic drugs for controlling blood glucose level.
Minor phenolics from Angelica keiskei and their proliferative effects on Hep3B cells.[Pubmed:28571822]
Bioorg Med Chem Lett. 2017 Jul 15;27(14):3065-3070.
A new coumarin, (-)-cis-(3'R,4'R)-4'-O-angeloylkhellactone-3'-O-beta-d-glucopyranoside (1) and two new chalcones, 3'-[(2E)-5-carboxy-3-methyl-2-pentenyl]-4,2',4'-trihydroxychalcone (4) and (+/-)-4,2',4'-trihydroxy-3'-2-hydroxy-2-[tetrahydro-2-methyl-5-(1-methylethenyl)-2-furanyl]ethylchalcone (5) were isolated from the aerial parts of Angelica keiskei (Umbelliferae), together with six known compounds: (R)-O-isobutyroyllomatin (2), 3'-O-methylvaginol (3), (-)-jejuchalcone F (6), isoliquiritigenin (7), Davidigenin (8), and (+/-)-liquiritigenin (9). The structures of the new compounds were determined by interpretation of their spectroscopic data including 1D and 2D NMR data. All known compounds (2, 3, and 6-9) were isolated as constituents of A. keiskei for the first time. To identify novel hepatocyte proliferation inducer for liver regeneration, 1-9 were evaluated for their cell proliferative effects using a Hep3B human hepatoma cell line. All isolates exhibited cell proliferative effects compared to untreated control (DMSO). Cytoprotective effects against oxidative stress induced by glucose oxidase were also examined on Hep3B cells and mouse fibroblast NIH3T3 cells and all compounds showed significant dose-dependent protection against oxidative stress.
The comparison of neuroprotective effects of isoliquiritigenin and its Phase I metabolites against glutamate-induced HT22 cell death.[Pubmed:27815122]
Bioorg Med Chem Lett. 2016 Dec 1;26(23):5639-5643.
It is becoming increasingly important to investigate drug metabolites to evaluate their toxic or preventive effects after administration of the parent compound. In our previous study, isoliquiritigenin isolated from Glycyrrhizae Radix effectively protected mouse-derived hippocampal neuronal cells (HT22) against 5mM glutamate-induced oxidative stress. However, there is little information on the protective effects of the metabolites of isoliquiritigenin on HT22 cells. In this study, isoliquiritigenin and its Phase I metabolites were prepared and their neuroprotective activities on glutamate-treated HT22 cells were compared. The prepared metabolites were liquiritigenin (1), 2',4,4',5'-tetrahydroxychalcone (2), sulfuretin (3), butein (4), Davidigenin (5), and cis-6,4'-dihydroxyaurone (6). Among the six metabolites, 4 showed better neuroprotective effects than the parent compound, isoliquiritigenin. Our study suggests that the neuroprotective effect of isoliquiritigenin could be elevated by its active metabolite 4, which is a chalcone containing a catechol group in the B ring.
Ethanol extract and isolated constituents from artemisia dracunculus inhibit esophageal squamous cell carcinoma and induce apoptotic cell death.[Pubmed:25076224]
Drug Res (Stuttg). 2015 Feb;65(2):101-6.
The objective of the present study was to examine the antitumor efficacy of the ethanol extract from Artemisia dracunculus as well as the compounds isolated from it on cultured EC‑109 esophageal squamous cell carcinoma (ESCC) cells. Apoptotic activities of the compounds were also studied using flow cytometry. EC‑109 esophageal cancer cells were treated with varying concentrations of compounds 1-7 isolated from the plant as well as the ethanol extract of Artemisia dracunculus. The cytotoxicity was evaluated by MTT assay and the apoptotic studies of the compounds were determined using flow-cytometry. Effect on mitochondrial membrane potential loss LambdaPsi m induced by compounds 2 and 4 was also studied in these cells. Bioassay-guided fractionation of the ethanol extract from the shoot and root parts of Artemisia dracunculus led to the isolation of 7-methoxycoumarin (1), scopoletin (2), dracumerin (3), sakuranetin (4), elimicin (5), Davidigenin (6) and 6-methoxycapillarisin (7). All the compounds as well as the extract showed mild to potent cell proliferation inhibitory activities against the esophageal cell line. Sakuranetin and 6-methoxycapillarisin were found to have the most potent effects in inhibiting the cell proliferation. The 2 potent compounds, sakuranetin and 6-methoxycapillarisin were evaluated for their effects on cell cycle phase distribution (DNA damage) as well as their effects on mitochondrial membrane potential loss LambdaPsi m. Both compounds induced DNA damage as well as mitochondrial membrane potential loss in esophageal cancer cells. The study suggests that compounds, Sakuranetin and 6-methoxycapillarisin isolated from Artemisia dracunculus possess potent anticancer effects by inducing DNA damage in these cells.
[C-ring cleavage of liquiritigenin extracted from licorice roots by an oxygen-tolerant bovine rumen bacterium strain Aeroto-Niu-O16].[Pubmed:22812014]
Yao Xue Xue Bao. 2012 May;47(5):664-9.
Aeroto-Niu-O16, an oxygen-tolerant bovine rumen bacterium, is capable of aerobically reducing isoflavones daidzein and genistein to dihydrodaidzein and dihydrogenistein through catalytic hydrogenation. In this study, it was found that bacterium strain Aeroto-Niu-O16 was able to cleavage the C-ring of liquiritigenin (LG), which is one of the main biologically active components of licorice roots, in the presence of atmospheric oxygen. LG was prepared by acid hydrolysis of the crude extract of licorice roots. The metabolite of LG obtained in strain Aeroto-Niu-O16 was identified as Davidigenin (DG) based on the data of UV, MS, 1H and 13C NMR. The maximal concentration of LG that the strain Aeroto-Niu-O16 was able to transform effectively was 0.8 mmol x L(-1) and the average productivity of the metabolite DG was 71.7%. Furthermore, when 0.1% (m/v) of L-cysteine or sodium thiosulfate was added in the cultural medium, the average bioconversion rate of LG was increased from 71.7% to 78.3% and 77.2%, respectively. The in vitro antioxidant investigation showed that 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity of DG was significantly or extremely significantly higher than that of LG at the concentrations from 0.2 mmol x L(-1) to 1.6 mmol x L(-1). We discoverd for the first time that LG can be converted to DG, which has stronger and wider biological activities, through microbial biotransformation method.
Dihydrochalcone glucosides and antioxidant activity from the roots of Anneslea fragrans var. lanceolata.[Pubmed:22459967]
Phytochemistry. 2012 Jun;78:120-5.
Bioassay-guided fractionation of the roots of Anneslea fragrans var. lanceolata led to the isolation of four dihydrochalcone glucosides, Davidigenin-2'-O-(6''-O-4'''-hydroxybenzoyl)-beta-glucoside (1), Davidigenin-2'-O-(2''-O-4'''-hydroxybenzoyl)-beta-glucoside (2), Davidigenin-2'-O-(3''-O-4'''-hydroxybenzoyl)-beta-glucoside (3), and Davidigenin-2'-O-(6''-O-syringoyl)-beta-glucoside (4), and 13 known compounds. The structures were identified by means of spectroscopic analysis. Davidigenin-2'-O-(6''-O-syringoyl)-beta-glucoside (4), 1-O-3,4-dimethoxy-5-hydroxyphenyl-6-O-(3,5-di-O-methylgalloyl)-beta-glucopyranoside (5), lyoniresinol (10), and syringic acid (13) showed ABTS [2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] cation radical scavenging activity, with SC(50) values of 52.6 +/- 5.5, 26.0 +/- 0.7, 6.0 +/- 0.2, and 27.5 +/- 0.6 mug/mL in 20 min, respectively. Lyoniresinol (10), isofraxidin (12), and syringic acid (13) also showed DPPH [1,1-diphenyl-2-picrylhydrazyl] radical scavenging activity, with SC(50) values of 8.4 +/- 1.8, 51.6 +/- 2.2, and 4.3 +/- 0.7 mug/mL in 30 min, respectively.
Qualitative variation of anti-diabetic compounds in different tarragon (Artemisia dracunculus L.) cytotypes.[Pubmed:21798321]
Fitoterapia. 2011 Oct;82(7):1062-74.
Ethanolic extracts of diploid Artemisia dracunculus L. (wild tarragon) from populations in the U.S., and polyploid tarragon from a variety of sources, were screened for the anti-diabetic compounds Davidigenin; sakuranetin; 2',4'-dihydroxy-4-methoxydihydrochalcone; 4,5-di-O-caffeoylquinic acid; 5-O-caffeoylquinic acid and 6-demethoxycapillarisin using LC-MS. Only decaploid plants contained all six target compounds and were the only plants that contained Davidigenin and 2,4-dihydroxy-4-methoxydihydrochalcone. These results exhibit the importance of germplasm selection and provenance when studying plants for medicinal activity. Relying only on the "right species" for consistent medicinal activities may not be sufficient, as intraspecific variation may be highly significant.
Antispasmodic and antioxidant activities of fractions and bioactive constituent davidigenin isolated from Mascarenhasia arborescens.[Pubmed:20580662]
J Ethnopharmacol. 2010 Jul 20;130(2):320-8.
ETHNOPHARMACOLOGICAL RELEVANCE: Mascarenhasia arborescens A. DC. (Apocynaceae) is used in traditional medicine in the North of Madagascar to treat intestinal disorders, intestinal spasms and diarrhoea. AIM OF THE STUDY: The main objective of this work was to evaluate the antispasmodic activity of the crude methanolic extract of Mascarenhasia arborescens and of its four partitions and to identify the effective compound responsible for this effect. MATERIALS AND METHODS: Isolation and structure elucidation techniques were performed in order to identify the bioactive constituent of Mascarenhasia arborescens and HPLC analysis was used for its quantification. Total phenolic content (TPC) of crude extracts and partitions were determined using the Folin-Ciocalteu method. Crude methanolic extract, partitions and the bioactive compound were investigated for their spasmolytic activity on several isolated organs. Their antiradical activity was also investigated by the DPPH test. RESULTS: Bioassay-guided fractionation using isolated guinea pig ileum pre-contracted with histamine 3x10(-6) M led to the isolation of Davidigenin (DG), a dihydrochalcone, as the main active constituent from the most promising methylene chloride partition (McP). This partition was effective on isolated guinea pig ileum pre-contracted with 3x10(-6) M histamine, with a median effective concentration (EC(50)) of 41.19+/-3.74 microg/ml. The DG content of this partition was shown to be 26.5% by HPLC. DG induced a concentration-dependent relaxation of the histamine pre-contracted guinea pig ileum with an EC(50) of 8.04+/-0.81 microg/ml and a concentration-dependent relaxation of the acetylcholine pre-contracted rat duodenum with an EC(50) of 9.35+/-0.30 microg/ml. It inhibited in a non-competitive manner histamine-induced isolated ileum contraction and the acetylcholine-induced isolated duodenum contraction. Moreover, DG does not have any antiradical activity. CONCLUSIONS: We demonstrated for the first time antispasmodic and antioxidant effects of Mascarenhasia arborescens. This study supports its use in traditional medicine. Furthermore, we highlighted the crucial role of Davidigenin in the antispasmodic activity of this plant.
Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus.[Pubmed:16806328]
Phytochemistry. 2006 Jul;67(14):1539-46.
An ethanolic extract of Artemisia dracunculus L. having antidiabetic activity was examined as a possible aldose reductase (ALR2) inhibitor, a key enzyme involved in diabetic complications. At 3.75 microg/mL, the total extract inhibited ALR2 activity by 40%, while quercitrin, a known ALR2 inhibitor, inhibited its activity by 54%. Bioactivity guided fractionation and isolation of the compounds that inhibit ALR2 activity was carried out with the total ethanolic extract yielding four bioactive compounds with ALR2 inhibitory activity ranging from 58% to 77% at 3.75 microg/mL. Using LC/MS, (1)H NMR, (13)C NMR and 2D NMR spectroscopic analyses, the four compounds were identified as 4,5-di-O-caffeoylquinic acid, Davidigenin, 6-demethoxycapillarisin and 2',4'-dihydroxy-4-methoxydihydrochalcone. This is the first report on their isolation from A. dracunculus and the ALR2 inhibitory activity of 4,5-di-O-caffeoylquinic acid, 6-demethoxycapillarisin and 2',4'-dihydroxy-4-methoxydihydrochalcone. These results suggest a use of the extract of A. dracunculus for ameliorating diabetic complications.


