Praeroside ICAS# 121064-73-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 121064-73-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C28H30O13 | M.Wt | 574.5 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Praeroside I Dilution Calculator

Praeroside I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7406 mL | 8.7032 mL | 17.4064 mL | 34.8129 mL | 43.5161 mL |
| 5 mM | 0.3481 mL | 1.7406 mL | 3.4813 mL | 6.9626 mL | 8.7032 mL |
| 10 mM | 0.1741 mL | 0.8703 mL | 1.7406 mL | 3.4813 mL | 4.3516 mL |
| 50 mM | 0.0348 mL | 0.1741 mL | 0.3481 mL | 0.6963 mL | 0.8703 mL |
| 100 mM | 0.0174 mL | 0.087 mL | 0.1741 mL | 0.3481 mL | 0.4352 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cryptostigmin II
Catalog No.:BCX2136
CAS No.:50906-57-5
- Davidigenin
Catalog No.:BCX2135
CAS No.:23130-26-9
- Pinocembrin 7-O-neohesperidoside
Catalog No.:BCX2134
CAS No.:13241-31-1
- Gerardianin A
Catalog No.:BCX2133
CAS No.:137171-31-4
- Euphopiloside A
Catalog No.:BCX2132
CAS No.:1610615-04-7
- Graciliflorin F
Catalog No.:BCX2131
CAS No.:1413941-67-9
- Eugenol 4-O-beta-D-(6'-O-galloyl) glucopyranoside
Catalog No.:BCX2130
CAS No.:152041-15-1
- Plumbagic acid
Catalog No.:BCX2129
CAS No.:75640-06-1
- Etuycomanol
Catalog No.:BCX2128
CAS No.:84633-28-3
- Ellagic acid glucoside
Catalog No.:BCX2127
CAS No.:163774-64-9
- Isorhamnetin 3-sambubioside
Catalog No.:BCX2126
CAS No.:142059-76-5
- 3'-Methoxynyasin
Catalog No.:BCX2125
CAS No.:1685246-20-1
- Quercetin-5-O-glucoside-3-O-rutinoside
Catalog No.:BCX2138
CAS No.:1478622-04-6
- Terrestriamide
Catalog No.:BCX2139
CAS No.:157536-49-7
- Auranamide
Catalog No.:BCX2140
CAS No.:740813-53-0
- 5-Allyl-1-methoxy-2,3-dihydroxybenzene
Catalog No.:BCX2141
CAS No.:4055-72-5
- Notoginsenoside E
Catalog No.:BCX2142
CAS No.:193976-50-0
- Hydrastinine
Catalog No.:BCX2143
CAS No.:5936-29-8
- 15-O-Methylgraciliflorin F
Catalog No.:BCX2144
CAS No.:1411994-51-8
- 4-Hydroxy-2-methoxybenzoic acid
Catalog No.:BCX2145
CAS No.:90111-34-5
- Mangostanin
Catalog No.:BCX2146
CAS No.:463342-39-4
- Monoisovalerate
Catalog No.:BCX2147
CAS No.:95486-32-1
- Ajugamarin A1
Catalog No.:BCX2148
CAS No.:78798-40-0
- Obtusichromoneside B
Catalog No.:BCX2149
CAS No.:2414481-39-1
Inhibitory effects of coumarin and acetylene constituents from the roots of Angelica furcijuga on D-galactosamine/lipopolysaccharide-induced liver injury in mice and on nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages.[Pubmed:16226032]
Bioorg Med Chem. 2006 Jan 15;14(2):456-63.
The methanolic extract (200 mg/kg, p.o. and i.p.), principal coumarin constituents (isoepoxypteryxin, anomalin, and Praeroside IV), and a polyacetylene constituent (falcarindiol) (25 mg/kg, i.p.) from the roots of Angelica furcijuga protected the liver injury induced by D-galactosamine (D-GalN)/lipopolysaccharide (LPS) in mice. In in vitro experiments, coumarin constituents (hyuganins A-D, anomalin, pteryxin, isopteryxin, and suksdorfin) and polyacetylene constituents [(-)-falcarinol and falcarindiol] substantially inhibited LPS-induced NO and/or TNF-alpha production in mouse peritoneal macrophages, and isoepoxypteryxin inhibited D-GalN-induced cytotoxicity in primary cultured rat hepatocytes. Furthermore, hyuganin A, anomalin, and isopteryxin inhibited the decrease in cell viability by TNF-alpha in L929 cells.
Antioxidant compounds from the leaves of Peucedanum japonicum thunb.[Pubmed:12926867]
J Agric Food Chem. 2003 Aug 27;51(18):5255-61.
Seventeen compounds were isolated from the n-butanol soluble fraction of the leaves of Peucedanum japonicum Thunb. On the basis of MS and various NMR spectroscopic techniques, the structures of the isolated compounds were determined as isoquercitrin (1), rutin (2), 3-O-caffeoylquinic acid (3), 4-O-caffeoylquinic acid (4), 5-O-caffeoylquinic acid (5), cnidioside A (6), Praeroside II (7), Praeroside III (8), apterin (9), esculin (10), (R)-peucedanol (11), (R)-peucedanol 7-O-beta-d-glucopyranoside (12), l-tryptophan (13), uracil (14), guanosine (15), uridine (16), and thymidine (17). All compounds except 11 and 12 were isolated for the first time from P. japonicum. Several isolated compounds were quantified by high-performance liquid chromatography analysis. In addition, all isolated compounds were examined for radical scavenging on 1,1-diphenyl-2-picrylhydrazyl radical and for inhibition of oxidation of liposome induced by 2,2'-azobis(2-amidinopropane)dihydrochloride. Compounds 2-5 were found to be the major potent constituents, which contribute to the antioxidant activity of P. japonicum leaves.
Hepatoprotective and nitric oxide production inhibitory activities of coumarin and polyacetylene constituents from the roots of Angelica furcijuga.[Pubmed:9873511]
Bioorg Med Chem Lett. 1998 Aug 18;8(16):2191-6.
The methanolic extract from the roots of Angelica furcijuga KITAGAWA was found to exhibit protective effects on liver injury induced by D-galactosamine (D-GalN) and lipopolysaccharide (LPS). From the methanolic extract, seventeen coumarins, two phenylpropanoids, and two polyacetylenes were isolated and examined their in vitro and in vivo hepatoprotective effects and inhibitory activity of NO production in macrophages. A acylated khellactone, isoepoxypteryxin, showed protective activity against D-GalN-induced cytotoxicity in primary cultured rat hepatocytes. On the other hand, six acylated khellactones (hyuganins A, B, C, and D, anomalin, isopteryxin) and two polyacetylenes [(-)-falcarinol and falcarindiol] strongly inhibited NO production induced by LPS in cultured mouse peritoneal macrophages, and also other acylated khellactones (isoepoxypteryxin, pteryxin, and suksdorfin) and a coumarin glycosides (Praeroside II) were found to show the activity. By comparison of the inhibitory activities for acylated khellactones with those for other coumarins, acyl groups were found to be essential to exerting potent activity.
Structures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu.[Pubmed:17262257]
Planta Med. 1989 Feb;55(1):64-7.
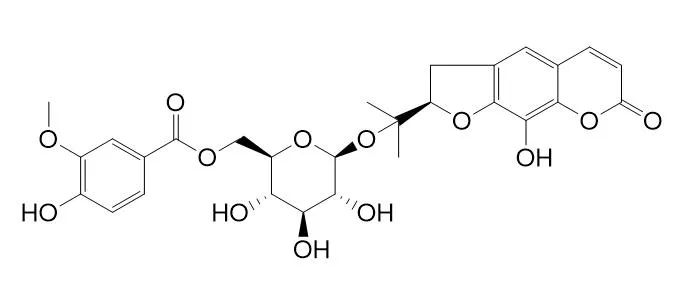
From the n-butanol extract of the crude drug "Bai-Hua Qian-Hu", the root of PEUCEDANUM PRAERUPTORUM Dunn. (Umbelliferae) Praeroside I ( 1) and three known linear-type furanocoumarin glycosides, isorutarin ( 2), rutarin ( 3), and marmesinin ( 4), along with two known simple coumarin glycosides, scopolin ( 5) and skimmin ( 6), were isolated. The structure of Praeroside I was established as rutaretin-4'- O-(6-vanilloyl-beta- D-glucopyranoside) by spectroscopic and chemical methods.


