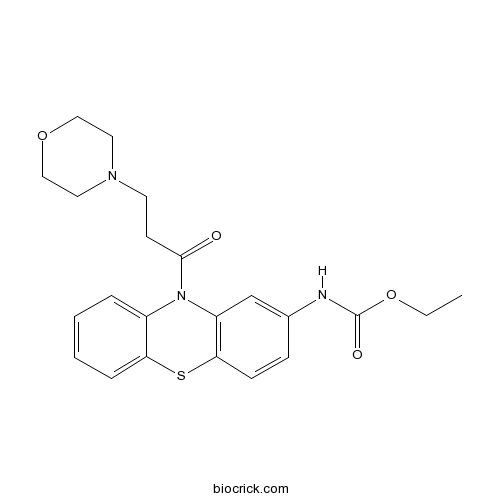
MoricizineCAS# 31883-05-3 |
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Docetaxel Trihydrate
Catalog No.:BCC1535
CAS No.:148408-66-6
- Colchicine
Catalog No.:BCN6271
CAS No.:64-86-8
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- 7-Xylosyltaxol
Catalog No.:BCN5341
CAS No.:90332-66-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31883-05-3 | SDF | Download SDF |
| PubChem ID | 34633 | Appearance | Powder |
| Formula | C22H25N3O4S | M.Wt | 427.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
| Chemical Name | ethyl N-[10-(3-morpholin-4-ylpropanoyl)phenothiazin-2-yl]carbamate | ||
| SMILES | CCOC(=O)NC1=CC2=C(C=C1)SC3=CC=CC=C3N2C(=O)CCN4CCOCC4 | ||
| Standard InChIKey | FUBVWMNBEHXPSU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H25N3O4S/c1-2-29-22(27)23-16-7-8-20-18(15-16)25(17-5-3-4-6-19(17)30-20)21(26)9-10-24-11-13-28-14-12-24/h3-8,15H,2,9-14H2,1H3,(H,23,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Moricizine Dilution Calculator

Moricizine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3391 mL | 11.6954 mL | 23.3907 mL | 46.7814 mL | 58.4768 mL |
| 5 mM | 0.4678 mL | 2.3391 mL | 4.6781 mL | 9.3563 mL | 11.6954 mL |
| 10 mM | 0.2339 mL | 1.1695 mL | 2.3391 mL | 4.6781 mL | 5.8477 mL |
| 50 mM | 0.0468 mL | 0.2339 mL | 0.4678 mL | 0.9356 mL | 1.1695 mL |
| 100 mM | 0.0234 mL | 0.117 mL | 0.2339 mL | 0.4678 mL | 0.5848 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- H-Phenylalaninol
Catalog No.:BCC2719
CAS No.:3182-95-4
- H-Phe-OEt.HCl
Catalog No.:BCC3007
CAS No.:3182-93-2
- Propranolol HCl
Catalog No.:BCC4336
CAS No.:318-98-9
- H-Ala-pNA.HCl
Catalog No.:BCC3195
CAS No.:31796-55-1
- (-)-Lyoniresinol
Catalog No.:BCN3488
CAS No.:31768-94-2
- 3-Deoxyaconitine
Catalog No.:BCN2797
CAS No.:3175-95-9
- 2-Methyl-4-(2-methylbenzoylamino)benzoic acid
Catalog No.:BCC8579
CAS No.:317374-08-6
- TCS 2314
Catalog No.:BCC6080
CAS No.:317353-73-4
- O-1602
Catalog No.:BCC7487
CAS No.:317321-41-8
- GW0742
Catalog No.:BCC2267
CAS No.:317318-84-6
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
- H-β-Ala-OMe.HCl
Catalog No.:BCC2853
CAS No.:3196-73-4
- Ethyl glucoside
Catalog No.:BCN5239
CAS No.:3198-49-0
- SIB 1757
Catalog No.:BCC6971
CAS No.:31993-01-8
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- Aclidinium Bromide
Catalog No.:BCC4575
CAS No.:320345-99-1
- Marmin acetonide
Catalog No.:BCN5240
CAS No.:320624-68-8
- Adenine sulfate
Catalog No.:BCC4451
CAS No.:321-30-2
Moricizine, an antiarrhythmic agent, as a potent inhibitor of hepatic microsomal CYP1A.[Pubmed:12393941]
Pharmacology. 2002 Dec;66(4):190-8.
We examined the inhibitory effect of Moricizine (MOR) on hepatic cytochrome P-450 (CYP) in mice. Spectrophotometric analysis revealed that MOR had a relatively high affinity for CYP molecules. MOR most potently inhibited the CYP1A1-dependent ethoxyresorufin O-deethylation and the CYP1A2-dependent methoxyresorufin O-demethylation, among the metabolic reactions mediated by CYP1A, CYP2A, CYP2B, CYP2C, CYP2D, CYP2E, and CYP3A subfamilies expressed in untreated and CYP-inducer-treated hepatic microsomes. The inhibition constants (K(i)) for ethoxyresorufin and methoxyresorufin O-dealkylations were 0.43 and 0.98 micromol/l, respectively. These K(i) values were one to three orders of magnitude lower than those of cimetidine (CIM) and mexiletine (MEX) that have been accepted as the clinical inhibitors of CYP1A2 and were below the therapeutic serum concentration of MOR. Theophylline 3-demethylation and 8-hydroxylation in untreated hepatic microsomes, clinical probes for CYP1A2 activities, were subjected to marked and competitive inhibition by MOR with K(i) values similar to that of methoxyresorufin O-demethylation, and the inhibitory potency of MOR was much higher than those of CIM and MEX. In addition, the zoxazolamine paralysis time, an in vivo measure of the hepatic CYP1A2 capacity, was markedly prolonged by pretreatment of mice with MOR rather than CIM and MEX, while the prolonging effect of MOR on the pentobarbital sleeping time, an indicator of the metabolic function of phenobarbital-inducible CYP species, was not so pronounced as compared with the zoxazolamine paralysis time. These results indicate that MOR acts as a potent and preferential inhibitor of hepatic CYP1A enzymes in vitro and in vivo.
Analysis of moricizine block of sodium current in isolated guinea-pig atrial myocytes. Atrioventricular difference of moricizine block.[Pubmed:12402511]
Vascul Pharmacol. 2002 Mar;38(3):131-41.
The effects of Moricizine on Na+ channel currents (INa) were investigated in guinea-pig atrial myocytes and its effects on INa in ventricular myocytes and on cloned hH1 current were compared using the whole-cell, patch-clamp technique. Moricizine induced the tonic block of INa with the apparent dissociation constant (Kd,app) of 6.3 microM at -100 mV and 99.3 microM at -140 mV. Moricizine at 30 microM shifted the h infinity curve to the hyperpolarizing direction by 8.6 +/- 2.4 mV. Moricizine also produced the phasic block of INa, which was enhanced with the increase in the duration of train pulses, and was more prominent with a holding potential (HP) of -100 mV than with an HP of -140 mV. The onset block of INa induced by Moricizine during depolarization to -20 mV was continuously increased with increasing the pulse duration, and was enhanced at the less negative HP. The slower component of recovery of the Moricizine-induced INa block was relatively slow, with a time constant of 4.2 +/- 2.0 s at -100 mV and 3.0 +/- 1.2 s at -140 mV. Since Moricizine induced the tonic block of ventricular INa with Kd,app of 3.1 +/- 0.8 microM at HP = -100 mV and 30.2 +/- 6.8 microM at HP = -140 mV, and cloned hH1 with Kd,app of 3.0 +/- 0.5 microM at HP = -100 mV and 22.0 +/- 3.2 microM at HP = -140 mV, respectively, either ventricular INa or cloned hH1 had significantly higher sensitivity to Moricizine than atrial INa. The h infinity curve of ventricular INa was shifted by 10.5 +/- 3.5 mV by 3 microM Moricizine and that of hH1 was shifted by 5.0 +/- 2.3 mV by 30 microM Moricizine. From the modulated receptor theory, we have estimated the dissociation constants for the resting and inactivated state to be 99.3 and 1.2 microM in atrial myocytes, 30 and 0.17 microM in ventricular myocytes, and 22 and 0.2 microM in cloned hH1, respectively. We conclude that Moricizine has a higher affinity for the inactivated Na+ channel than for the resting state channel in atrial myocytes, and Moricizine showed the significant atrioventricular difference of Moricizine block on INa. Moricizine would exert an antiarrhythmic action on atrial myocytes, as well as on ventricular myocytes, by blocking Na+ channels with a high affinity to the inactivated state and a slow dissociation kinetics.
Moricizine induced increase in pacing threshold.[Pubmed:12685150]
Pacing Clin Electrophysiol. 2003 Jan;26(1 Pt 1):110-1.
A 72-year-old woman who was experiencing incessant ventricular tachycardia and recurrent automatic implantable cardioverter defibrillator (AICD) firing despite amiodarone therapy was referred to the Cleveland Clinic Foundation. Myocardial ischemia and infarction were ruled out by standard means. Several antiarrhythmic medications were tried previously without success. Moricizine, 200 mg three times daily, was initiated and controlled the ventricular tachycardia. However, after the dose of Moricizine was titrated upward, the patient became symptomatically bradycardic and the ECG exhibited 2:1 block of her paced rhythm and an increased ventricular pacing threshold.


