Aclidinium BromideLAMAs antagonist CAS# 320345-99-1 |
- Cevimeline
Catalog No.:BCC1470
CAS No.:107233-08-9
- Tolterodine tartrate
Catalog No.:BCC4586
CAS No.:124937-52-6
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Solifenacin succinate
Catalog No.:BCC4580
CAS No.:242478-38-2
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

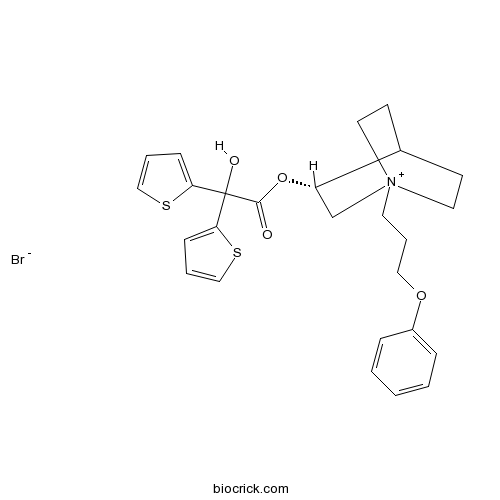
| Cas No. | 320345-99-1 | SDF | Download SDF |
| PubChem ID | 11519741 | Appearance | Powder |
| Formula | C26H30BrNO4S2 | M.Wt | 564.55 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | LAS 34273; LAS-W 330 | ||
| Solubility | DMSO : 33.33 mg/mL (59.04 mM; Need ultrasonic) | ||
| Chemical Name | [(3R)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octan-3-yl] 2-hydroxy-2,2-dithiophen-2-ylacetate;bromide | ||
| SMILES | C1C[N+]2(CCC1C(C2)OC(=O)C(C3=CC=CS3)(C4=CC=CS4)O)CCCOC5=CC=CC=C5.[Br-] | ||
| Standard InChIKey | XLAKJQPTOJHYDR-QTQXQZBYSA-M | ||
| Standard InChI | InChI=1S/C26H30NO4S2.BrH/c28-25(26(29,23-9-4-17-32-23)24-10-5-18-33-24)31-22-19-27(14-11-20(22)12-15-27)13-6-16-30-21-7-2-1-3-8-21;/h1-5,7-10,17-18,20,22,29H,6,11-16,19H2;1H/q+1;/p-1/t20?,22-,27?;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aclidinium Bromide(LAS 34273; LAS-W 330) is a long-acting, inhaled muscarinic antagonist as a maintenance treatment for chronic obstructive pulmonary disease (COPD).
IC50 value:
Target: M3 receptor
Preclinically, aclidinium showed potent antagonism of human muscarinic receptors, with a long residence time at M3 receptors and a shorter residence time at M2 receptors, indicating the potential to provide sustained bronchodilation. Aclidinium is rapidly hydrolysed in human plasma, unlike other currently available antimuscarinics including tiotropium. Early clinical studies in healthy subjects have confirmed the low systemic bioavailability and favourable safety profile of single and multiple doses of aclidinium. In a subsequent Phase IIb study, which included 464 patients with moderate to severe COPD, aclidinium displayed long-lasting bronchodilatory activity and was well tolerated. References: | |||||

Aclidinium Bromide Dilution Calculator

Aclidinium Bromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7713 mL | 8.8566 mL | 17.7132 mL | 35.4264 mL | 44.2831 mL |
| 5 mM | 0.3543 mL | 1.7713 mL | 3.5426 mL | 7.0853 mL | 8.8566 mL |
| 10 mM | 0.1771 mL | 0.8857 mL | 1.7713 mL | 3.5426 mL | 4.4283 mL |
| 50 mM | 0.0354 mL | 0.1771 mL | 0.3543 mL | 0.7085 mL | 0.8857 mL |
| 100 mM | 0.0177 mL | 0.0886 mL | 0.1771 mL | 0.3543 mL | 0.4428 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aclidinium Bromide is a long-acting antagonist of muscarinic (LAMAs) [1].
Aclidinium Bromide has been reported to inhibit the specific binding of [3H]-NMS to human M receptors in a dose-denpendent fasion with Ki values of 0.10±0.00nM,0.14±0.04nM, 0.14±0.02nM, 0.21±0.04nM and 0.16±0.01nM for M1, M2,M3,M4 and M5, respectively. In addition, Aclidinium Bromide has shown the great potency at endogenous M2 and M3 receptors with EC50 values of 17.4±1.1nM and 5.3±1.6nM, respectively. Apart from these, Aclidinium Bromide has been revealed to produce a concentration-dependent inhibition of acetylcholine-induced bronchoconstriction in vivo with an EC50 of ranging from 2.5 to 23.1μg/mL in anaesthetized guines pigs. Besides, Aclidinium Bromide has also been noted to inhibit the salivation in rat pilocarpine-induced sialorrhea model with an EC50 of 38μg/kg [1].
References:
[1] Gavaldà A1, Ramos I2, Carcasona C3, Calama E4, Otal R5, Montero JL6, Sentellas S7, Aparici M8, Vilella D9, Alberti J10, Beleta J11, Miralpeix M12. The in vitro and in vivo profile of aclidinium bromide in comparison with glycopyrronium bromide. Pulm Pharmacol Ther. 2014 Aug;28(2):114-21.
- 5-Azacytidine
Catalog No.:BCC1130
CAS No.:320-67-2
- SIB 1757
Catalog No.:BCC6971
CAS No.:31993-01-8
- Ethyl glucoside
Catalog No.:BCN5239
CAS No.:3198-49-0
- H-β-Ala-OMe.HCl
Catalog No.:BCC2853
CAS No.:3196-73-4
- Norarmepavine
Catalog No.:BCN7077
CAS No.:3195-01-5
- Cornucervine
Catalog No.:BCN1962
CAS No.:31948-48-8
- Axitinib (AG 013736)
Catalog No.:BCC3729
CAS No.:319460-85-0
- (RS)-3-Hydroxyphenylglycine
Catalog No.:BCC6604
CAS No.:31932-87-3
- H-D-Allo-Ile-OH
Catalog No.:BCC2683
CAS No.:319-78-8
- Moricizine
Catalog No.:BCC5235
CAS No.:31883-05-3
- Methyl orsellinate
Catalog No.:BCN5238
CAS No.:3187-58-4
- H-Orn-OH. HCl
Catalog No.:BCC3000
CAS No.:3184-13-2
- Marmin acetonide
Catalog No.:BCN5240
CAS No.:320624-68-8
- Adenine sulfate
Catalog No.:BCC4451
CAS No.:321-30-2
- Fluoronaphthalene
Catalog No.:BCC8987
CAS No.:321-38-0
- EO 1428
Catalog No.:BCC7511
CAS No.:321351-00-2
- [D-Trp8]-γ-MSH
Catalog No.:BCC7902
CAS No.:321351-81-9
- Bellendine
Catalog No.:BCN1895
CAS No.:32152-73-1
- Z- Pyr-OH
Catalog No.:BCC3330
CAS No.:32159-21-0
- N-Acetyl-4-piperidone
Catalog No.:BCC9079
CAS No.:32161-06-1
- Cytosporone B
Catalog No.:BCN6791
CAS No.:321661-62-5
- BIBR 1532
Catalog No.:BCC1147
CAS No.:321674-73-1
- Poloxin
Catalog No.:BCC1867
CAS No.:321688-88-4
- L002
Catalog No.:BCC8000
CAS No.:321695-57-2
Effect of aclidinium bromide on cough and sputum symptoms in moderate-to-severe COPD in three phase III trials.[Pubmed:28074135]
BMJ Open Respir Res. 2016 Dec 8;3(1):e000148.
BACKGROUND: Cough and sputum are troublesome symptoms in chronic obstructive pulmonary disease (COPD) and are associated with adverse outcomes. The efficacy of Aclidinium Bromide 400 microg twice daily in patients with stable COPD has been established in two phase III studies (ACCORD COPD I and ATTAIN) and a phase IIIb active-comparator study. This analysis evaluated cough-related symptoms across these studies. METHOD: Patients were randomised to placebo, aclidinium 200 microg or 400 microg twice daily in ACCORD (12 weeks) and ATTAIN (24 weeks), or to placebo, aclidinium 400 microg twice daily or tiotropium 18 microg once daily (6-week active-comparator study). Analysed end points included changes from baseline in Evaluating Respiratory Symptoms (E-RS; formerly known as EXAcerbations of Chronic pulmonary disease Tool), total and cough/sputum scores and frequency/severity of morning and night-time cough and sputum symptoms. RESULTS: Data for 1792 patients were evaluated. E-RS cough/sputum domain scores were significantly reduced with aclidinium 400 microg versus placebo in ATTAIN (-0.7 vs -0.3, respectively; p<0.01) and the active-comparator study (-0.6 vs -0.2, respectively; p<0.01). In the active-comparator study, significantly greater improvements were observed with aclidinium versus placebo for severity of morning cough (-0.19 vs -0.02; p<0.01) and phlegm (-0.19 vs -0.02; p<0.05). In ACCORD, aclidinium reduced night-time cough frequency (-0.36 vs 0.1 for placebo; p<0.001) and severity (-0.24 vs -0.1 for placebo; p<0.05), and frequency of night-time sputum production (-0.37 vs 0.05 for placebo; p<0.001). CONCLUSIONS: Aclidinium 400 microg twice daily improves cough and sputum expectoration versus placebo in stable COPD. TRIAL REGISTRATION NUMBERS: NCT00891462; NCT01001494; NCT01462929.
Cost versus utility of aclidinium bromide 400 microg plus formoterol fumarate dihydrate 12 microg compared to aclidinium bromide 400 microg alone in the management of moderate-to-severe COPD.[Pubmed:27672337]
Clinicoecon Outcomes Res. 2016 Sep 12;8:445-56.
PURPOSE: Aclidinium-formoterol 400/12 microg is a long-acting muscarinic antagonist (LAMA) and a long-acting beta2-agonist in a fixed-dose combination used in the management of patients with COPD. This study aimed to assess the cost-effectiveness of aclidinium-formoterol 400/12 microg against the long-acting muscarinic antagonist Aclidinium Bromide 400 microg. MATERIALS AND METHODS: A five-health-state Markov transition model with monthly cycles was developed using MS Excel to simulate patients with moderate-to-severe COPD and their initial lung-function improvement following treatment with aclidinium-formoterol 400/12 microg or aclidinium 400 microg. Health states were based on severity levels defined by Global Initiative for Chronic Obstructive Lung Disease 2010 criteria. The analysis was a head-to-head comparison without step-up therapy, from the NHS Scotland perspective, over a 5-year time horizon. Clinical data on initial lung-function improvement were provided by a pooled analysis of the ACLIFORM and AUGMENT trials. Management, event costs, and utilities were health state-specific. Costs and effects were discounted at an annual rate of 3.5%. The outcome of the analysis was expressed as cost (UK pound) per quality-adjusted life-year (QALY) gained. The analysis included one way and probabilistic sensitivity analyses to investigate the impact of parameter uncertainty on model outputs. RESULTS: Aclidinium-formoterol 400/12 microg provided marginally higher costs ( pound41) and more QALYs (0.014), resulting in an incremental cost-effectiveness ratio of pound2,976/QALY. Sensitivity analyses indicated that results were robust to key parameter variations, and the main drivers were: mean baseline forced expiratory volume in 1 second (FEV1), risk of exacerbation, FEV1 improvement from aclidinium-formoterol 400/12 microg, and lung-function decline. The probability of aclidinium-formoterol 400/12 microg being cost-effective (using a willingness-to-pay threshold of pound20,000/QALY) versus aclidinium 400 microg was 79%. CONCLUSION: In Scotland, aclidinium-formoterol 400/12 microg can be considered a cost-effective treatment option compared to aclidinium 400 microg alone in patients with moderate-to-severe COPD.


