L002p300 inhibitor CAS# 321695-57-2 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 321695-57-2 | SDF | Download SDF |
| PubChem ID | 2221149 | Appearance | Powder |
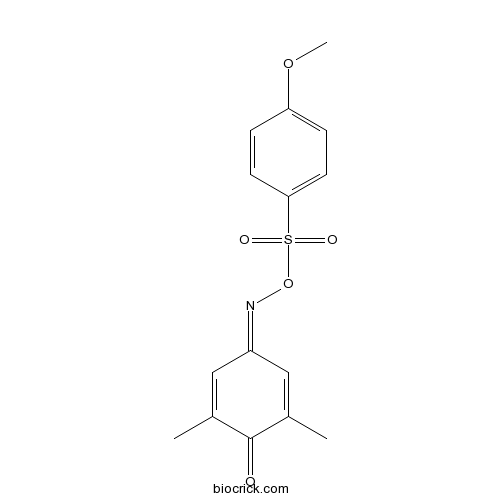
| Formula | C15H15NO5S | M.Wt | 321.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 10 mM in ethanol with gentle warming | ||
| Chemical Name | [(3,5-dimethyl-4-oxocyclohexa-2,5-dien-1-ylidene)amino] 4-methoxybenzenesulfonate | ||
| SMILES | CC1=CC(=NOS(=O)(=O)C2=CC=C(C=C2)OC)C=C(C1=O)C | ||
| Standard InChIKey | VEWFTYOFWIXCIO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H15NO5S/c1-10-8-12(9-11(2)15(10)17)16-21-22(18,19)14-6-4-13(20-3)5-7-14/h4-9H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | p300 inhibitor (IC50 = 1.98 μM). Inhibits histone and p53 acetylation, and suppresses STAT3 activation in cell-based assays. Also suppresses tumor growth in a mouse MDA-MB-468 xenograft model. |

L002 Dilution Calculator

L002 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1119 mL | 15.5594 mL | 31.1187 mL | 62.2374 mL | 77.7968 mL |
| 5 mM | 0.6224 mL | 3.1119 mL | 6.2237 mL | 12.4475 mL | 15.5594 mL |
| 10 mM | 0.3112 mL | 1.5559 mL | 3.1119 mL | 6.2237 mL | 7.7797 mL |
| 50 mM | 0.0622 mL | 0.3112 mL | 0.6224 mL | 1.2447 mL | 1.5559 mL |
| 100 mM | 0.0311 mL | 0.1556 mL | 0.3112 mL | 0.6224 mL | 0.778 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Poloxin
Catalog No.:BCC1867
CAS No.:321688-88-4
- BIBR 1532
Catalog No.:BCC1147
CAS No.:321674-73-1
- Cytosporone B
Catalog No.:BCN6791
CAS No.:321661-62-5
- N-Acetyl-4-piperidone
Catalog No.:BCC9079
CAS No.:32161-06-1
- Z- Pyr-OH
Catalog No.:BCC3330
CAS No.:32159-21-0
- Bellendine
Catalog No.:BCN1895
CAS No.:32152-73-1
- [D-Trp8]-γ-MSH
Catalog No.:BCC7902
CAS No.:321351-81-9
- EO 1428
Catalog No.:BCC7511
CAS No.:321351-00-2
- Fluoronaphthalene
Catalog No.:BCC8987
CAS No.:321-38-0
- Adenine sulfate
Catalog No.:BCC4451
CAS No.:321-30-2
- Marmin acetonide
Catalog No.:BCN5240
CAS No.:320624-68-8
- Aclidinium Bromide
Catalog No.:BCC4575
CAS No.:320345-99-1
- Pilloin
Catalog No.:BCN6817
CAS No.:32174-62-2
- 1,10:4,5-Diepoxy-7(11)-germacren-8-one
Catalog No.:BCN1460
CAS No.:32179-18-3
- p-3-Methylamino propyl phenol
Catalog No.:BCN1802
CAS No.:32180-92-0
- Triflusal
Catalog No.:BCC4443
CAS No.:322-79-2
- Heraclenol 3'-O-beta-D-glucopyranoside
Catalog No.:BCN1459
CAS No.:32207-10-6
- H-Asp(OMe)-OMe.HCl
Catalog No.:BCC2890
CAS No.:32213-95-9
- Lupeol palmitate
Catalog No.:BCN7133
CAS No.:32214-80-5
- Calcitriol
Catalog No.:BCC4950
CAS No.:32222-06-3
- 2alpha,7beta,13alpha-Triacetoxy-5alpha-cinnamoyloxy-9beta-hydroxy-2(3->20)abeotaxa-4(20),11-dien-10-one
Catalog No.:BCN7208
CAS No.:322471-42-1
- SCH 221510
Catalog No.:BCC7612
CAS No.:322473-89-2
- Methyl 3-cyclopropyl-3-oxopropionate
Catalog No.:BCC9038
CAS No.:32249-35-7
- 4-Methoxy-1-methylquinolin-2-one
Catalog No.:BCN4824
CAS No.:32262-18-3
Acetyltransferase p300 inhibitor reverses hypertension-induced cardiac fibrosis.[Pubmed:30710427]
J Cell Mol Med. 2019 Apr;23(4):3026-3031.
Epigenetic dysregulation plays a crucial role in cardiovascular diseases. Previously, we reported that acetyltransferase p300 (ATp300) inhibitor L002 prevents hypertension-induced cardiac hypertrophy and fibrosis in a murine model. In this short communication, we show that treatment of hypertensive mice with ATp300-specific small molecule inhibitor L002 or C646 reverses hypertension-induced left ventricular hypertrophy, cardiac fibrosis and diastolic dysfunction, without reducing elevated blood pressures. Biochemically, treatment with L002 and C646 also reverse hypertension-induced histone acetylation and myofibroblast differentiation in murine ventricles. Our results confirm and extend the role of ATp300, a major epigenetic regulator, in the pathobiology of cardiac hypertrophy and fibrosis. Most importantly, we identify the efficacies of ATp300 inhibitors C646 and L002 in reversing hypertension-induced cardiac hypertrophy and fibrosis, and discover new anti-hypertrophic and anti-fibrotic candidates.
2D boron nitride nanosheets (BNNS) prepared by high-pressure homogenisation: structure and morphology.[Pubmed:30318554]
Nanoscale. 2018 Nov 7;10(41):19469-19477.
2D Boron Nitride Nano-sheets (BNNS) were prepared using a high-pressure homogenisation process to exfoliate bulk hexagonal boron nitride (h-BN). The effectiveness of this process was studied by characterising bulk h-BN and BNNS post-processing using numerous techniques. The BNNS produced was composed of a mixture of sheets having lengths on the nanometre (nm) scale, but lateral thicknesses on the micron (mum) length scale. The product was a macro-porous material containing slit-like pores with a surface area of 170 m(2) g(-1). It had a polycrystalline structure with d002 = 0.335 nm and L002 = 2 nm. From the sharp E2g peak in the Raman spectrum at 1360 cm(-1) (FWHM = 12.5 cm(-1)), the sheets had a low defect density and were highly exfoliated. X-Ray photoelectron spectroscopy (XPS) studies detected B-OH and N-H groups on the BNNS surface and the presence of residual surfactant. Contact angle measurements (60 degrees +/- 3 degrees (0 s); 40 degrees +/- 2 degrees (10 s)) confirmed a hydrophilic surface. The BNNS was thermally stable under oxidative conditions up to 323 degrees C.
Effect of a Histone Demethylase Inhibitor on Equine Herpesvirus-1 Activity In Vitro.[Pubmed:29594155]
Front Vet Sci. 2018 Mar 12;5:34.
Equine herpesvirus type 1 (EHV-1) is a ubiquitous and highly contagious pathogen that causes a range of disease severities with outbreaks of notable economic impact. Given the limitations in immune protection of current vaccines and the limited effectiveness of antiviral drugs on EHV-1 infections in vivo, improved treatment measures are needed to control disease. The use of drugs that alter the epigenetic state of herpes simplex virus genome has been shown to limit viral primary infection and reactivation both in vitro and in vivo. Therefore, we tested the hypothesis that maintaining a repressive epigenetic state on the EHV-1 genome in the host equine cell would decrease viral load during lytic infection. Equine fetal kidney cells (EFKCs) or isolated peripheral blood leukocytes were treated in vitro with (a) the nucleoside analog ganciclovir; (b) the histone demethylase inhibitor OG-L002; (c) both ganciclovir and OG-L002; or (d) dimethyl sulfoxide (DMSO, vehicle control); and then infected with a clinical EHV-1 isolate. Treatment of EFKCs with ganciclovir (mean 22.3 DNA copies per cell, p = 0.0005), OG-L002 (mean 25.6, p = 0.005) or both ganciclovir and OG-L002 (mean 7.1, p = 0.0001) resulted in decreased EHV-1 viral load at 24 h post-infection (hpi) in comparison with DMSO (mean 42.0), with greater impact using the combined treatment. Further, EHV-1 gene expression at 3 hpi decreased when EFKCs were infected in the presence of ganciclovir (p = 0.04) and combined treatment of ganciclovir and OG-L002 (p = 0.0003). In contrast, under similar conditions, neither ganciclovir nor OG-L002 suppressed EHV-1 infection in leukocytes. Differences between cell types, drug penetrance, or drug turnover, may have contributed to the distinct effects observed in this study.
A novel acetyltransferase p300 inhibitor ameliorates hypertension-associated cardio-renal fibrosis.[Pubmed:28933600]
Epigenetics. 2017;12(11):1004-1013.
Hypertension-associated end-organ damage commonly leads to cardiac and renal fibrosis. As no effective anti-fibrotic therapy currently exists, the unchecked progression of fibrogenesis manifests as cardio-renal failure and early death. We have previously shown that FATp300-p300 with intrinsic factor acetyltransferase activity-is an essential epigenetic regulator of fibrogenesis, and is elevated in several fibrotic tissues. In this report, we investigate the therapeutic efficacy of a novel FATp300 inhibitor, L002, in a murine model of hypertensive cardio-renal fibrosis. Additionally, we examine the effects of L002 on cellular pro-fibrogenic processes and provide mechanistic insights into its antifibrogenic action. Utilizing cardiac fibroblasts, podocytes, and mesangial cells, we demonstrate that L002 blunts FATp300-mediated acetylation of specific histones. Further, incubating cells with L002 suppresses several pro-fibrogenic processes including cellular proliferation, migration, myofibroblast differentiation and collagen synthesis. Importantly, systemic administration of L002 in mice reduces hypertension-associated pathological hypertrophy, cardiac fibrosis and renal fibrosis. The anti-hypertrophic and anti-fibrotic effects of L002 were independent of blood pressure regulation. Our work solidifies the role of epigenetic regulator FATp300 in fibrogenesis and establishes it as a pharmacological target for reducing pathological matrix remodeling and associated pathologies. Additionally, we discover a new therapeutic role of L002, as it ameliorates hypertension-induced cardio-renal fibrosis and antagonizes pro-fibrogenic responses in fibroblasts, podocytes and mesangial cells.
Role of the DNA repair glycosylase OGG1 in the activation of murine splenocytes.[Pubmed:28843610]
DNA Repair (Amst). 2017 Oct;58:13-20.
OGG1 (8-oxoguanine-DNA glycosylase) is the major DNA repair glycosylase removing the premutagenic DNA base modification 8-oxo-7,8-dihydroguanine (8-oxoG) from the genome of mammalian cells. In addition, there is accumulating evidence that OGG1 and its substrate 8-oxoG might function in the regulation of certain genes, which could account for an attenuated immune response observed in Ogg1(-/-) mice in several settings. Indications for at least two different mechanisms have been obtained. Thus, OGG1 could either act as an ancillary transcription factor cooperating with the lysine-specific demethylase LSD1 or as an activator of small GTPases. Here, we analysed the activation by lipopolysaccaride (LPS) of primary splenocytes obtained from two different Ogg1(-/-) mouse strains. We found that the induction of TNF-alpha expression was reduced in splenocytes (in particular macrophages) of both Ogg1(-/-) strains. Notably, an inhibitor of LSD1, OG-L002, reduced the induction of TNF-alpha mRNA in splenocytes from wild-type mice to the level observed in splenocytes from Ogg1(-/-) mice and had no influence in the latter cells. In contrast, inhibitors of the MAP kinases p38 and JNK as well as the antioxidant N-acetylcysteine attenuated the LPS-stimulated TNF-alpha expression both in the absence and presence of OGG1. The free base 8-oxo-7,8-dihydroguanine had no influence on the TNF-alpha expression in the splenocytes. The data demonstrate that OGG1 plays a role in an LSD1-dependent pathway of LPS-induced macrophage activation in mice.
Small-molecule inhibitors of acetyltransferase p300 identified by high-throughput screening are potent anticancer agents.[Pubmed:23625935]
Mol Cancer Ther. 2013 May;12(5):610-20.
Acetyltransferase p300 (KAT3B) plays key roles in signaling cascades that support cancer cell survival and sustained proliferation. Thus, p300 represents a potential anticancer therapeutic target. To discover novel anticancer agents that target p300, we conducted a high-throughput screening campaign. A library of 622,079 compounds was assayed for cytotoxicity to the triple-negative breast cancer (TNBC) cell line MDA-MB-231 but not to the human mammary epithelial cells. The resulting compounds were tested in a biochemical assay for inhibiting the enzymatic activity of p300. One compound (L002, NSC764414) displayed an IC50 of 1.98 mumol/L against p300 in vitro, inhibited acetylation of histones and p53, and suppressed STAT3 activation in cell-based assays. L002 could be docked to the active site of the p300 catalytic domain. Biochemical tests of a series of related compounds revealed functional groups that may impact inhibitory potency of L002 against p300. Interestingly, these analogs showed inhibitory activities against the cellular paralog of p300 (CBP), p300/CBP-associated factor, and GCN5, but not to other acetyltransferases (KAT5, KAT6B, and KAT7), histone deacetylases, and histone methyltransferases. Among the NCI-60 panel of cancer cell lines, leukemia and lymphoma cell lines were extremely sensitive to L002, whereas it is toxic to only a limited number of cell lines derived from solid tumors. Notably, breast cancer cell lines, especially those derived from TNBC, were highly susceptible to L002. In vivo, it potently suppressed tumor growth and histone acetylation of MDA-MB-468 xenografts. Thus, these new acetyltransferase inhibitors are potential anticancer therapeutics.


