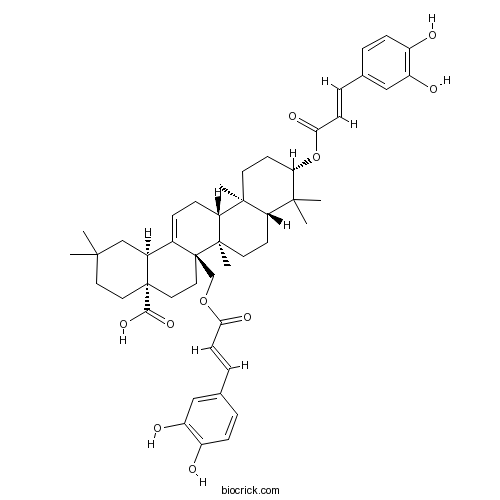
Myriceric acid CCAS# 162059-94-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 162059-94-1 | SDF | Download SDF |
| PubChem ID | 15767725 | Appearance | Powder |
| Formula | C48H60O10 | M.Wt | 797.0 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4aS,6aR,6aR,6bR,8aR,10S,12aR,14bS)-10-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-6a-[[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxymethyl]-2,2,6b,9,9,12a-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)OC(=O)C=CC6=CC(=C(C=C6)O)O)C)C)C2C1)COC(=O)C=CC7=CC(=C(C=C7)O)O)C(=O)O)C | ||
| Standard InChIKey | OQOHHYHCCUJBHE-HQEPTUIISA-N | ||
| Standard InChI | InChI=1S/C48H60O10/c1-43(2)21-22-47(42(55)56)23-24-48(28-57-40(53)15-9-29-7-12-33(49)35(51)25-29)31(32(47)27-43)11-14-38-45(5)19-18-39(44(3,4)37(45)17-20-46(38,48)6)58-41(54)16-10-30-8-13-34(50)36(52)26-30/h7-13,15-16,25-26,32,37-39,49-52H,14,17-24,27-28H2,1-6H3,(H,55,56)/b15-9+,16-10+/t32-,37-,38+,39-,45-,46+,47-,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Myriceric acid C has cytotoxic activity against human lung carcinoma and breast carcinoma. |
| Targets | Endothelin Receptor |

Myriceric acid C Dilution Calculator

Myriceric acid C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2547 mL | 6.2735 mL | 12.5471 mL | 25.0941 mL | 31.3676 mL |
| 5 mM | 0.2509 mL | 1.2547 mL | 2.5094 mL | 5.0188 mL | 6.2735 mL |
| 10 mM | 0.1255 mL | 0.6274 mL | 1.2547 mL | 2.5094 mL | 3.1368 mL |
| 50 mM | 0.0251 mL | 0.1255 mL | 0.2509 mL | 0.5019 mL | 0.6274 mL |
| 100 mM | 0.0125 mL | 0.0627 mL | 0.1255 mL | 0.2509 mL | 0.3137 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- SC 58125
Catalog No.:BCC5948
CAS No.:162054-19-5
- L-371,257
Catalog No.:BCC7353
CAS No.:162042-44-6
- UNC0379
Catalog No.:BCC8055
CAS No.:1620401-82-2
- Rofecoxib
Catalog No.:BCC4437
CAS No.:162011-90-7
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
- GSK2801
Catalog No.:BCC6498
CAS No.:1619994-68-1
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- Catechin pentaacetate
Catalog No.:BCN1718
CAS No.:16198-01-9
- Esomeprazole Magnesium
Catalog No.:BCC5007
CAS No.:161973-10-0
- Prehelminthosporol
Catalog No.:BCN7447
CAS No.:1619-13-2
- Segetalin A
Catalog No.:BCC9246
CAS No.:161875-97-4
- Talampanel(LY300164)
Catalog No.:BCC6378
CAS No.:161832-65-1
- Dimesna
Catalog No.:BCC1095
CAS No.:16208-51-8
- 4',4'''-Di-O-methylisochamaejasmin
Catalog No.:BCN6849
CAS No.:1620921-68-7
- SynaptoRedTM C2
Catalog No.:BCC8012
CAS No.:162112-35-8
- RWJ 50271
Catalog No.:BCC7894
CAS No.:162112-37-0
- Flunitrazepam
Catalog No.:BCC6107
CAS No.:1622-62-4
- Melperone hydrochloride
Catalog No.:BCC7385
CAS No.:1622-79-3
- 5'-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine 2',3'-diacetate
Catalog No.:BCN1544
CAS No.:162204-20-8
- Dorsmanin A
Catalog No.:BCN4088
CAS No.:162229-27-8
- CI 1020
Catalog No.:BCC7523
CAS No.:162256-50-0
- 7,3'-Dihydroxy-4'-methoxyflavan
Catalog No.:BCN4698
CAS No.:162290-05-3
- Remodelin
Catalog No.:BCC5571
CAS No.:1622921-15-6
- Baccatin X
Catalog No.:BCN7214
CAS No.:1623069-76-0
Endothelin receptor antagonist triterpenoid, myriceric acid A, isolated from Myrica cerifera, and structure activity relationships of its derivatives.[Pubmed:8998841]
Chem Pharm Bull (Tokyo). 1996 Feb;44(2):343-51.
As the first non-peptide endothelin receptor antagonist from a higher plant, a new triterpenoid, myriceric acid A (50-235) (1) was isolated from the bayberry, Myrica cerifera. Myriceric acid A (1) inhibited not only an endothelin-1-induced increase in cytosolic free Ca2+ concentration (IC50 = 11 +/- 2 nM) but [125I]endothelin-1 binding in rat aortic smooth muscle cells (Ki = 66 +/- 15 nM). Two new related triterpenoids, Myriceric acid C (6), and myriceric acid D (8), were also isolated. Furthermore, the chemical modification of these natural products led to the synthesis of sulfated derivatives (13, 14, 15) which showed 1.5 to 20 times higher affinity for endothelin receptors. The structure activity relationships of myriceric acids and their derivatives are discussed.
Constituents from the stems of Hibiscus taiwanensis.[Pubmed:15635230]
Chem Pharm Bull (Tokyo). 2005 Jan;53(1):56-9.
Five new compounds, hibicuslide A (1), hibicuslide B (2), hibicuslide C (3), hibicutaiwanin (4), hibicusin (5), and fifty-one known compounds have been isolated from the stems of Hibiscus taiwanensis. The structures of these compounds were determined by spectroscopic and chemical transformation methods. Among them, mansonone H (19) and uncarinic acid A (30) inhibited HIV replication in H9 lymphocyte cells. The 9,9'-O-feruloyl-(-)-secoisolaricinresinol (12), Myriceric acid C (29), and uncarinic acid A (30) showed cytotoxic activity against human lung carcinoma and breast carcinoma.


